| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |28 |29 |30 |31 |32 |33 |34 |35 |36 |37 |38 |39 |40 |41 |42 |43 |44 |review |
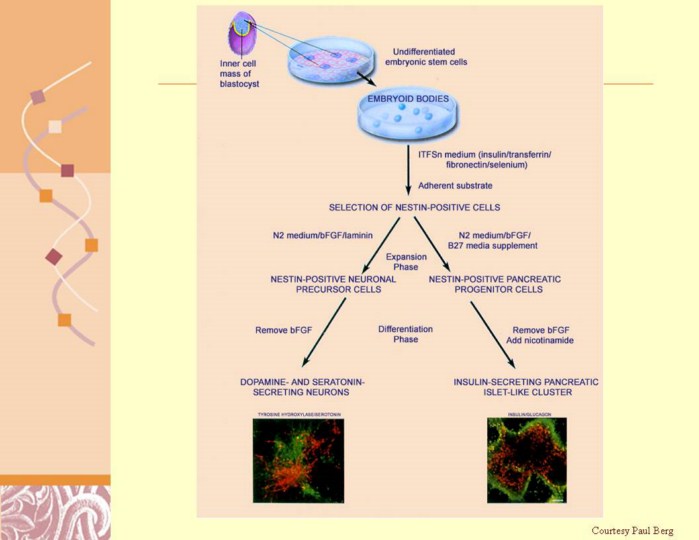 |
Fibronectin is a high-molecular weight (~440kDa) extracellular matrix glycoprotein that binds to membrane-spanning receptor proteins called integrins.[1] In addition to integrins, fibronectin also binds extracellular matrix components such as collagen, fibrin and heparan sulfate proteoglycans (e.g. syndecans). Fibronectin exists as a dimer, consisting of two nearly identical monomers linked by a pair of disulfide bonds.[1] The fibronectin protein is produced from a single gene, but alternative splicing of its pre-mRNA leads to the creation of several isoforms.
Nestin is a type VI intermediate filament (IF) protein.[1][2] These intermediate filament proteins are expressed mostly in nerve cells where they are implicated in the radial growth of the axon. Seven genes encode for the heavy (NF-H), medium (NF-M) and light neurofilament (NF-L) proteins, nestin and α-internexin in nerve cells, synemin α and desmuslin/synemin β (two alternative transcripts of the DMN gene) in muscle cells, and syncoilin (also in muscle cells). Members of this group mostly preferentially coassemble as heteropolymers in tissues. Steinert et al. has shown that nestin forms homodimers and homotetramers but does not form IF by itself in vitro. In mixtures, nestin preferentially co-assembles with purified vimentin or the type IV IF protein -internexin to form heterodimer coiled-coil molecules.[3]
Laminins are major proteins in the basal lamina (formerly improperly called "basement membrane"), a protein network foundation for most cells and organs. The laminins are an important and biologically active part of the basal lamina, influencing cell differentiation, migration, adhesion as well as phenotype and survival.[1] Laminins are great trimeric proteins that contain an α-chain, a β-chain, and a γ-chain, found in five, three, and three genetic variants, each. The laminin molecules are named according to their chain composition. Thus, laminin-511 contains α5, β1, and γ1 chains.[2] Fourteen other chain combinations have been identified in vivo. The trimeric proteins form a plus sign with one long arm, giving a structure that can bind to other cell membrane and extracellular matrix molecules.[3] The three shorter arms are particularly good at binding to other laminin molecules, which allows them to form sheets. The long arm is capable of binding to cells, which helps anchor organized tissue cells to the membrane. The laminins are a family of glycoproteins that are an integral part of the structural scaffolding in almost every tissue of an organism. They are secreted and incorporated into cell-associated extracellular matrices. Laminin is vital for the maintenance and survival of tissues. Defective laminins can cause muscles to form improperly, leading to a form of muscular dystrophy, lethal skin blistering disease (junctional epidermolysis bullosa) and defects of the kidney filter
Transferrin is a blood plasma protein for iron delivery that, in humans, is encoded by the TF gene.[1] Transferrin is a glycoprotein that binds iron very tightly but reversibly. Although iron bound to transferrin is less than 0.1% (4 mg) of the total body iron, it is the most important iron pool, with the highest rate of turnover (25 mg/24 h). Transferrin has a molecular weight of around 80 kDa and contains 2 specific high-affinity Fe(III) binding sites. The affinity of transferrin for Fe(III) is extremely high (1023 M−1 at pH 7.4)[2] but decreases progressively with decreasing pH below neutrality. |