| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |28 |29 |30 |31 |32 |33 |34 |35 |36 |37 |38 |39 |40 |41 |42 |43 |44 |review |
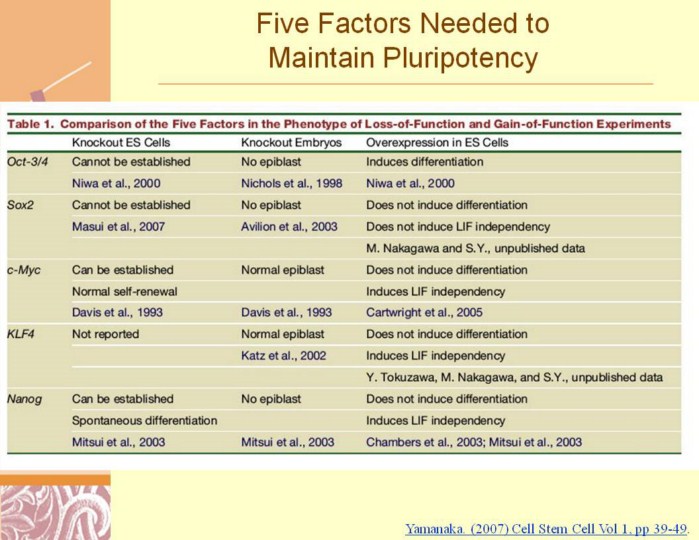 |
Yamanaka. (2007) Cell Stem Cell Vol 1, pp 39-49
Oct-3/4 was identified as a novel Oct family protein specifically expressed in EC cells, early embryos, and germ cells (Okamoto et al., 1990; Rosner et al., 1990; Scholer et al., 1990). The Oct family transcription factors contain the POU domain, an 150 amino acid sequence conserved among Pit-l, Oct-1, Oct-2, and uric-86. Oct-3/4 and other POU proteins bind to the octamer sequence (ATTA/TGCAT). Expression of Oct-3/4 is restricted in the blastomeres of the developing mouse embryo, the ICM of blastocysts, the epiblast, and germ cells. It is also expressed in pluripotent stem cells, including ES cells, EG cells, EC cells, and mGS cells
Sox2 was identified as a Sox (SRY-related HMG box) protein expressed in EC cells (Yuan et al., 1995). The high mobility group (HMG) domain is a DNA binding domain conserved in abundant chromosomal proteins including HMG1 and HMG2, which bind DNA with little or no sequence specificity, and in sequence-specific transcription factors, including SRY, SOX, and LEF-1 All SOX factors appear to recognize a similar binding motif, A/TA/TCAAA/TG. Like Oct-3/4, Sox2 also marks the pluripotent lineage of the early mouse embryo; expressed in the ICM, epiblast, and germ cells. Unlike Oct-3/4, however, Sox2 is also expressed by the multipotential cells of the extraembryonic ectoderm (Avilion et al., 2003). In addition, Sox2 expression is associated with uncommitted dividing stem and precursor cells of the developing central nervous system (CNS), and it can be used to isolate such cells (Li et al., 1998; Zappone et al., 2000).
c-Myc is one of the first proto-oncogenes found in human cancers (Dalla-Favera et al., 1982). The N terminus of Myc binds to several proteins, including TRRAP, which are components of the TIP60 and GCN5 histone acetyltransferase complexes, and TIP48 and TIP49, which contain ATPase domains (Adhikary and Eilers, 2005). The C terminus of the Myc protein contains the basic region/helixloop- helix/leucine zipper (BR/HLH/LZ) domain, through which Myc binds to a partner protein, Max. The Myc- Max dimers bind to a DNA sequence (CACA/GTG), which is a subset of the general E box sequence (CANNTG) that is bound by all bHLH proteins. In addition to binding to DNA, the C terminus of Myc is also involved in transactivation through binding to CBP and p300, which have histone acetylase activities. |