 |
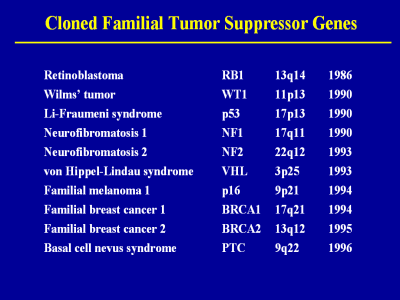
Most of the syndromes that were initially
linked to cancer were due to tumor suppressor genes and they often involved
the intramural program at NCI. After the germline mutations were identified
for retinoblastoma, Wilms tumor, and Li-Fraumeni syndrome, the NCI and NINDS
contributed families with neurofibromatosis 1 and 2 to groups that
eventually cloned the genes. The clinical and laboratory studies of Marston
Linehan and Burt Zbar culminated in their discovery of several genes
responsible for hereditary forms of renal cancer, starting with von
Hippel-Lindau syndrome. The work of Peggy Tucker and Alisa Goldstein in
hereditary melanoma led to their discovery of germline mutations of the
tumor suppressor gene CDKN2A, and later the proto-oncogene CDK4. Our studies
of familial breast cancer focused in the Ashkenazi Jewish population shed
light on the role of founder mutations of BRCA 1 and BRCA 2. In addition,
our families with the multiple basal cell carcinoma syndrome were studied by
Mike Dean, who uncovered germline mutations of the patch gene. Since 1996
when I stopped making this slide, more than 50 inherited gene mutations
involving tumor suppressors, proto-oncogenes, and mismatched repair genes
have been identified through studies of these hereditary syndromes. And this
information has greatly enhanced our understanding of the genetic and
molecular mechanisms that are involved both in cancer etiology and
progression. |
