| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |28 |29 |30 |31 |32 |33 |34 |35 |36 |37 |38 |39 |40 |41 |42 |43 |44 |45 |review |
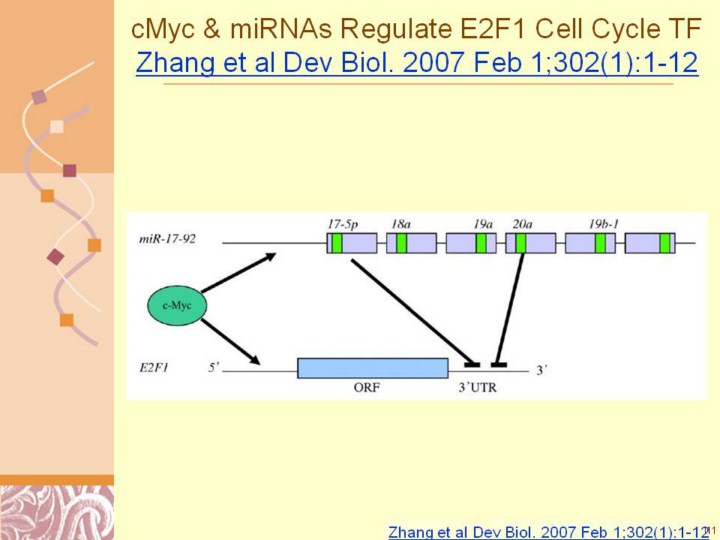 |
Zhang et al Dev Biol. 2007 Feb 1;302(1):1-12 Modulation of cell cycle transcription factor E2F1 by a c-Myc-regulated microRNA polycistron miR-17–92 and its function in cancer pathogenesis (O'Donnell et al., 2005). The upper part of the panel shows the schematic representation of the microRNA polycistron miR-17–92 cluster. The larger boxes represent the miRNA precursors (pre-miRNAs), and the smaller boxes within represent the mature miRNAs. c-Myc promotes the transcription of both miR-17–92 and E2F1 by binding at the CACGTG or CARGTG sites on the miR-17–92 gene (O'Donnell et al., 2005) and promoter sites of E2F1. Both miR-17-5p and miR-20a negatively regulate E2F1 gene expression through translational repression by directly binding miR-17-5p and miR-20a at the 3′UTR of E2F1 mRNAs. Level expression results in the inhibition of apoptosis through the ARF-p53 pathway, and causes cancer pathogenesis in several organs, including lung cancer and lymphomas. In this regulatory mechanism, miR-17–92 functions as an oncogene. |