| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |28 |29 |30 |31 |32 |33 |34 |35 |36 |37 |38 |39 |40 |41 |42 |43 |44 |45 |46 |47 |48 |49 |review |
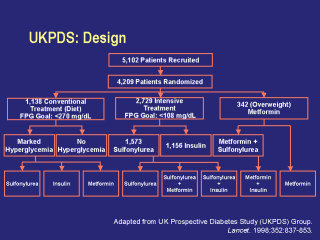 |
This multicenter, randomized, controlled trial
sought to determine whether: intensive glycemic control (study goal: fasting plasma glucose [FPG] <108 mg/dL) reduces the risk of microvascular and macrovascular complications of type 2 diabetes sulfonylurea, metformin (in obese patients), or insulin has specific advantages or disadvantages tight blood pressure control (<150/85 mm Hg) reduces the risk of complications tight blood pressure control with an ACE inhibitor or a beta-blocker has specific advantages in preventing the microvascular and macrovascular complications of diabetes. Of the 7,616 patients referred to the UKPDS, 5,102 were enrolled in a 3-month dietary run-in phase during which they followed a diet that was low in saturated fats, was of moderately high fiber, and that derived 50% of calories from carbohydrates. The other 2,514 patients were excluded from the study. After completion of the dietary phase, 744 patients were excluded because their FPG was >270 mg/dL and an additional 149 were excluded because of FPG levels that were <=108 mg/dL. The remaining patients (4,209) were stratified by ideal body weight and randomized to conventional or intensive treatment. Conventional treatment consisted of initial dietary therapy only, with a goal FPG of <270 mg/dL. If the goal was not met, subjects were randomized to receive a sulfonylurea, insulin, or metformin. The FPG goal remained the same. Intensive treatment consisted of a sulfonylurea (either chlorpropamide, glibenclamide, or glipizide) or insulin, with a goal of maintaining FPG <108 mg/dL. Intensive metformin therapy was an additional treatment option for overweight patients. In the intensive treatment group, combination therapy was initiated when glycemic goals were not achieved with monotherapy. For those patients who had been receiving a sulfonylurea, metformin or insulin was added; metformin-treated patients had a sulfonylurea added if the glycemic goal was not met; and insulin was substituted for a sulfonylurea if necessary to achieve the glycemic goal. Aggregate endpoints included: any diabetes-related clinical endpoint (sudden death, death from hyperglycemia or hypoglycemia, fatal or nonfatal MI, angina, heart failure, stroke, renal failure, amputation of at least one digit, vitreous hemorrhage, retinal photocoagulation, blindness in an eye, or cataract extraction) diabetes-related death (death from an MI, stroke, peripheral vascular disease (PVD), renal disease, hyperglycemia or hypoglycemia, or sudden death)all-cause mortality. UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837-853. |