 |
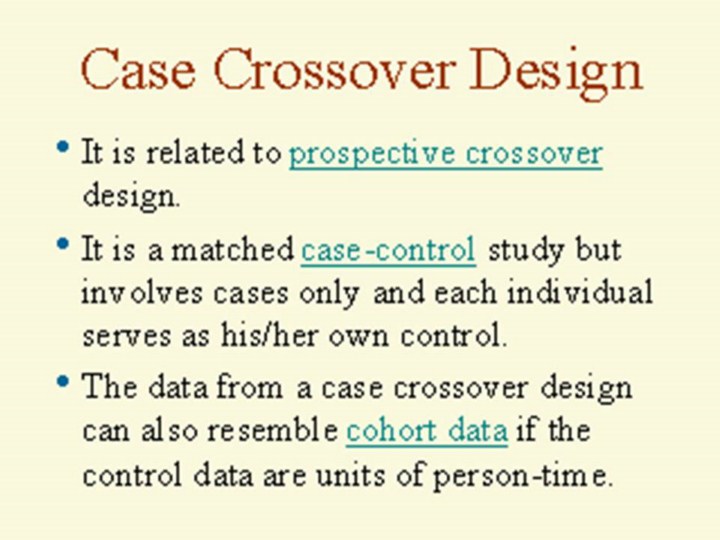
- The
term "crossover" is mainly used to describe experiments in which all
individuals pass through both the treatment and placebo phases. In the case
crossover design, the treatment phase is the hazard period and placebo phase
is the control period. Since there are a hazard period and a control period
and each individual provides the exposure information for both hazard and
control periods, case crossover design can be viewed as a matched
case-control study design where it involves cases only and each individual
serves as his/her own control.
- On the other hand, the case crossover study can also be viewed as a
retrospective cohort study as the control data are not necessarily the
"counts", they can be units of exposure person-time (this will be
illustrated in slides 13, 16, 20). When it is this case, the analysis for
the study can be treated as a pooled analysis of several cohort studies each
with a sample size of one.
|
