| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |review |
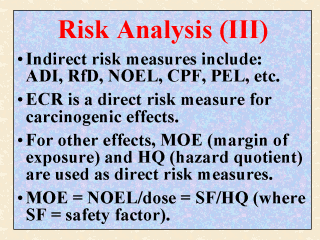 |
Those measures
or terms used to characterize health risk indirectly include: ADI (acceptable daily
intake), RfD (reference dose), RfC (reference concentration), PEL (permissible limit
level), CPF (cancer potency factor), and such. These indirect risk measures were discussed
in the last two slides. They are considered here as indirect risk measures primarily
because their values must be checked by (against) the exposure or the dose in question
before the risk can be assessed and characterized. ECR (excess cancer risk), as discussed in the last slide, is considered a direct risk measure because no further comparison with an estimated dose is needed. For non-carcinogenic adverse effects, the direct risk measures commonly used by regulatory agencies are the MOE, HQ, and some variants of these two. Until recently, MOE (margin of exposure) was used interchangeably with MOS (margin of safety). It is determined by dividing the NOEL by the dose in question. HQ (hazard quotient), on the other hand, is the ratio of the dose to the pre-established RfD (or RfC). The main difference between MOE and HQ is that the latter can be used as is, since a safety factor (SF) has been incorporated to derive the RfD (or RfC). Thus, a HQ value of 1 or less is considered safe. On the other hand, an acceptable MOE (also called a benchmark MOE) is typically 100 or greater in order to account for an uncertainty factor of 10 for interspecies difference and another 10 for intraspecies sensitivity. Given that MOE = (NOEL)/(dose), HQ = (dose)/(RfD), and RfD = (NOEL)/(SF), it follows that MOE = (SF)/(HQ). |