| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |review |
 |
To date, there
have been at least a dozen terms or measures used to denote directly or indirectly a
health risk as (in)significant. These risk measures include, but certainly are not limited
to: ADI, RfD, RfC, CPF, ECR, PEL, TLV, STEL, BMD, BMC, MCL, NOEL, NOAEL, LOEL. Of these,
the first 3 would have their values built in with a safety margin. Closely related to
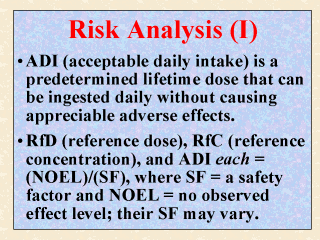
these dozen or more terms are MOE and HQ. ADI (acceptable daily intake, typically expressed in mg/kg/day) is the predetermined amount of a chemical (e.g., pesticide, food additive) that presumably can be ingested daily by humans for an entire lifetime without causing appreciable adverse effects. This dosage is determined by dividing the NOEL by a safety factor (SF), usually of multiple of 10, to account for intraspecies sensitivity, interspecies difference, and other factors of concern (e.g. completeness of data). The definitions of NOEL, NOAEL, and LOEL were given in Slide 8. The term ADI was first used by the joint Food and Agriculture Organization/World Health Organization Expert Committee on Food Additives in 1961, and is now used extensively by USFDA (Lu, 1988). RfD (reference dose), used by many programs within USEPA, is also derived similarly. The difference between RfD and ADI is the possible different SF used by the (two) regulatory agencies. RfC (reference concentration) is also determined and used similarly by USEPA. It is different from RfD only in that chemical (air) concentration, rather than the actual dose, is considered. (Other terms not elaborated here are discussed in the slides that follow.) |