| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |28 |29 |30 |31 |32 |33 |34 |35 |36 |37 |38 |39 |40 |41 |review |
 |
Few head-to-head trials have compared the
various tobacco cessation therapies. In a randomized controlled trial
comparing the four NRT formulations available at the time, the products
performed similarly, but patient compliance was higher with the patch,
followed by the gum, which was higher than the inhaler and nasal spray
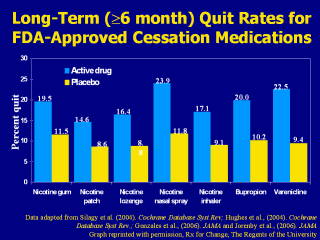
(Hajek et al., 1999). This bar chart summarizes the long-term (≥6-month) quit rates observed with the different NRT products, bupropion SR and varenicline (Gonzales et al., 2006; Hughes et al., 2004; Jorenby et al., 2006; Silagy et al., 2004). These data derive from 124 different placebo-controlled trials; therefore, it is inappropriate to compare the active medications with respect to clinical efficacy. What this chart does illustrate, however, is that the quit rates from each of the methods is approximately twice that of its corresponding placebo control treatment arm. Each of the pharmacotherapy options depicted in the chart is considered effective. When patients ask for assistance with their quit attempt, any product can be recommended, if not contraindicated. However, when assisting patients in choosing a product, clinicians should consider additional factors. The number of cigarettes smoked per day (or time to first cigarette, for the nicotine lozenge), level of dependence, advantages and disadvantages of each product, methods used for prior quit attempts and reasons for relapse, and the patient’s own product preference need to be considered. Behavioral counseling should be used in conjunction with all pharmacologic therapies. Gonzales D, Rennard SI, Nides M, et al. (2006). Varenicline, an ?4ß2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 296:47-55. Hajek P, West R, Foulds J, Nilsson F, Burrows S, Meadow A. (1999). Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med 159:2033–2038. Hughes JR, Stead LF, Lancaster. (2004). Antidepressants for smoking cessation. Cochrane Database Syst Rev 4:CD000031. Jorenby DE, Hays JT, Rigotti NA, et al. (2006). Efficacy of varenicline, an α4ß2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 296:56-63. Silagy C, Lancaster T, Stead L, Mant D, Fowler G. (2004). Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 3:CD000146. Data adapted from Silagy et al. (2004). Cochrane Database Syst Rev; Hughes et al., (2004). Cochrane Database Syst Rev.; Gonzales et al., (2006). JAMA and Jorenby et al., (2006). JAMA Data adapted from Silagy et al. (2004). Cochrane Database Syst Rev;
Hughes et al., (2004). Cochrane Database Syst Rev.; Gonzales et al., (2006).
JAMA and Jorenby et al., (2006). JAMA |