| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |review |
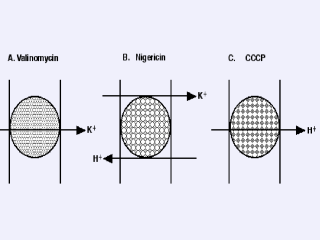 |
Figure 8. Ionophores. Ionophores are small, lipid soluble molecules that allow specific ions to permeate the membrane. They are classified as (i) mobile carriers [e.g., valinomycin, nigericin, 2,4-dinitrophenol (DNP) or carbonylcyanide-m-chlorophenylhydrazone (CCCP)] that carry 103-104 ions/carrier/sec and (ii) channels (e.g., gramicidin) that move 107 ions/channel/sec which will not be discussed. Many ionophores were discovered due to their antibacterial properties. A dehydrated K+ ion is coordinated precisely and specifically to carbonyl groups in the hydrophilic interior of valinomycin (A) whose exterior is nonpolar and thereby soluble in the membrane. Because valinomycin is neutral, it carries the single (+) charge of the bound K+ through the membrane (i.e., it is electrogenic). Thus, it can be used either to generate DY in response to a K+ gradient or to dissipate DY if flux of another ion (e.g., protons) is generating DY. Nigericin (B) catalyzes the electrically neutral exchange of 1 proton for 1 K+ (preferentially over Na+). Therefore, this ionophore will dissipate DpH specifically. Alternatively, it can be used to convert a K+ gradient into a DpH. DNP or CCCP (C) are lipid soluble weak acids that permeabilize the membrane to protons specifically, thereby collapsing both DY and DpH. |