 |
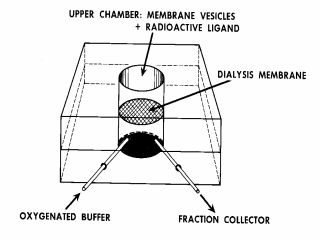
Figure 6. Quantitative measurement of concentration gradients by flow
dialysis. As mentioned above, the filtration assay for accumulation is
relatively quantitative for charged and neutral substrates, but
accumulation of weak acids, in particular, is drastically underestimated
because of high passive permeability in the protonated state. This causes
accumulated weak acids to leak rapidly from the vesicles during dilution and
filtration. Therefore, flow dialysis is used. By this means, the
concentration of any small molecular weight solute in the medium surrounding
the vesicles can be measured continuously without manipulation. A flow
dialysis unit consists of a Lucite block with 2 chambers separated by a
dialysis membrane and inlet and outlet ports in the lower chamber through
which oxygenated buffer is pumped to either a fraction collector or a
continuous flow scintillation counter. The basic idea is that vesicles
in the upper chamber are much too large to pass through the dialysis
membrane. In contrast, a radiolabeled weak acid or base, a lipophilic ion
or a sugar, amino acid or another transported substrate in the external
medium can pass through the dialysis membrane. Therefore, the
concentration of the radiolabeled compound in the solution bathing the
vesicles in the upper chamber is measured continuously by monitoring the
solution flowing out of the lower chamber. If the vesicles accumulate
the radiolabeled material in the upper chamber, the dialyzable concentration
drops which is reflected by a decrease in radioactivity in the flow-through
from the lower chamber. Conversely, if the radiolabeled compound
accumulated by the vesicles is released, the level of radioactivity in the
flow-through increases. The technique is extremely powerful, as it
allows quantification of concentration gradients of the probes used to
measure DY or
DpH, as well as transported
substrates under identical conditions. |
