| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |review |
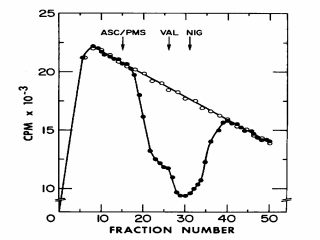 |
Figure 7. A typical flow dialysis experiment in which accumulation of [14C]acetate is used to measure DpH (interior alkaline) in RSO membrane vesicles from E. coli. At zero time, [14C]acetate is added to the upper chamber, and the effluent from the lower chamber is monitored continuously for radioactivity. In all cases, radioactivity rapidly appears in the lower chamber, reaches a maximum at about 10 min and then slowly decreases due to dialysis and continuous flow of solution from the lower chamber. Open circles: [14C]acetate added to buffer in the upper chamber in the absence of membrane vesicles; closed circles, [14C]acetate added to the upper chamber in the presence of vesicles. If [14C]acetate were bound non-specifically to the vesicles, the dialyzable concentration of [14C]acetate would decrease in the presence of vesicles because the dialyzable concentration of the weak acid would decrease. Thus, there is little or no non-specific binding of [14C]acetate. Upon addition of ascorbate and phenazine methosulfate (ASC/PMS; ê), a dramatic decrease in the concentration of [14C]acetate in the dialysate is observed which reflects accumulation of the weak acid by the vesicles in the upper chamber, and a new equilibrium is reached at about 10,000 CPM lower than the control. At this point, valinomycin, an ionophore that collapses DY by allowing rapid influx of K+ is added (VAL; ê). The concentration of [14C]acetate in the dialysate decreases even further, indicating that internal pH has become even more alkaline, thereby increasing DpH (interior alkaline). Finally, nigericin which collapses DpH by exchanging one proton for one K+ or Na+ across the membrane is added (NIG; ê), and the concentration of [14C]acetate in the dialysate returns to the control level. |