 |
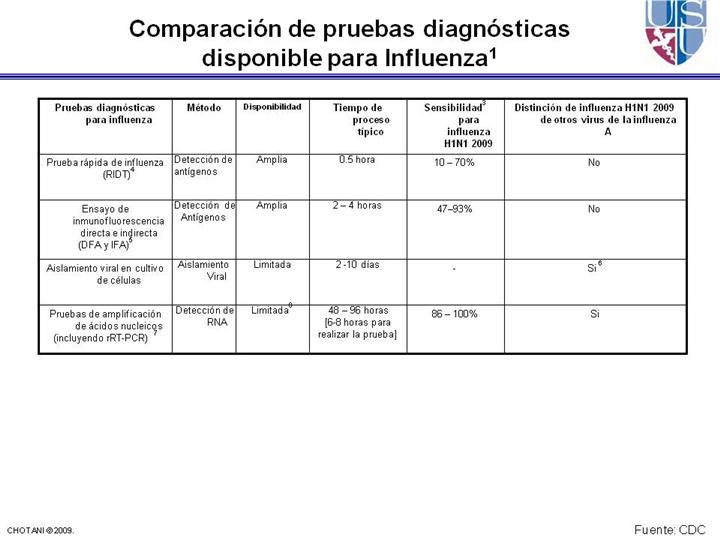
1 -
Serologic testing on paired acute- (within 1 week of illness onset) and
convalescent-phase (collected 2-3 weeks later) sera is limited to
epidemiological and research studies, is not routinely available through
clinical laboratories, and should not inform clinical decisions.
2 – Cantidad de tiempo necesaria desde la colección del especímen hasta
disponibilidad de resultados.
3 - Compared with rRT-PCR tests; rRT-PCR tests are compared to other testing
modalities including other rRT-PCR assays.
4 - Rapid Influenza Diagnostic Tests include tests that are CLIA waived (can
be performed in an outpatient setting) and tests that are moderately complex
(can be performed only in a laboratory). Clinical specimens approved for
RIDTs vary by test, and may not include all respiratory specimens.
5 - Performance of these assays relies heavily on laboratory expertise and
requires a fluorescent microscope
6 - Requires additional testing on the viral isolate
7 - The performance of rRT-PCR assays specific for 2009 H1N1 influenza have
not been established for bronchoalveolar lavage and tracheal aspirates. If
testing these specimens for 2009 H1N1 influenza consider testing in parallel
with a nasopharyngeal, nasal, or oropharyngeal swabs or a nasal aspirate.
8 - See discussion on available rRT-PCR assays at
http://www.cdc.gov/h1n1flu/guidance/diagnostic_tests.htm
|
