| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |review |
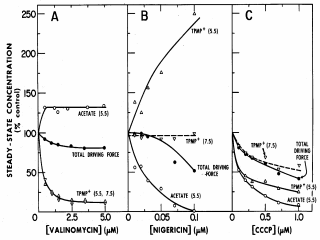 |
Figure 3. Effect of valinomycin, nigericin or CCCP on
DpH (acetate accumulation), DY
(TPMP+ accumulation) and
Panel A, Valinomycin. When increasing concentrations of valinomycin are added in the presence of K+ at either pH 5.5 or pH 7.5, TPMP+ decreases markedly, as expected (i.e., rapid K+ influx mediated by the ionophore neutralizes DY). Since DY and DpH are related reciprocally, DpH increases as DY decreases, and the total driving force hardly changes. Panel B, Nigericin. When increasing concentrations of nigericin are added at pH 5.5, acetate accumulation (DpH) decreases sharply, as expected due to exchange of K+ with protons, and DY increases reciprocally with a little or no change in the total driving force. At pH 7.5, where there is little or no DpH and no acetate accumulation, the ionophore has no effect on TPMP+ accumulation, as there is no DpH to convert into DY. Panel C, CCCP. Increasing concentrations of the protonophore CCCP decrease both acetate and TPMP+ accumulation at pH 5.5 and pH 7.5, demonstrating that the electrogenic ion is hydrogen. Since the pK of CCCP is about 6, the protonophore is more effective at pH 5.5 than at 7.5. The specific predictions of the Chemiosmotic Paradigm with respect to active transport can now be tested further. If active transport of weak acids is driven specifically by DpH, not only should there be no accumulation at pH 7.5, but valinomycin should stimulate accumulation at pH 5.5, just as it stimulates acetate accumulation. If active transport of cations involves uniport coupled specifically to DY, lysine accumulation should be inhibited by valinomycin at both pH 5.5 and 7.5, but stimulated by nigericin at pH 5.5 with no effect of the ionophore at pH 7.5. |