 |
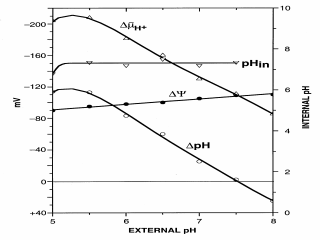
Figure 2. Effect of external pH on internal pH,
DpH,
DY and
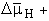 .
Since E. coli is a
so-called “neutrophile” that can grow over a pH range from about 5.5 to 8.5,
it is informative to measure these parameters as a function of external pH.
Internal pH remains constant at pH 7.6 from an external pH of about pH 5.0
to pH 7.6 when measured by using [14C]acetate and flow dialysis.
As a result, DpH is about –120 mV at
pH 5.0 and 5.5, but then decreases dramatically, and at pH 7.6, there is no
DpH because internal and external pH are equal. Above pH
7.6, internal pH becomes acidic relative to external pH (i.e., methylamine
is accumulated) although protons are being pumped out of the cell.
This behavior is due to the action of a sodium/proton antiporter (NhaA) that
exchanges one internal sodium ion for two external protons (i.e., it is
electrogenic), thereby acidifying the internal milieu, and the antiporter is
activated when the external pH rises above pH 7.6. Over the same pH range,
DY (as determined by TPP+
accumulation) rises from about –90 mV to about –110 mV. As a result of
these changes, .
Since E. coli is a
so-called “neutrophile” that can grow over a pH range from about 5.5 to 8.5,
it is informative to measure these parameters as a function of external pH.
Internal pH remains constant at pH 7.6 from an external pH of about pH 5.0
to pH 7.6 when measured by using [14C]acetate and flow dialysis.
As a result, DpH is about –120 mV at
pH 5.0 and 5.5, but then decreases dramatically, and at pH 7.6, there is no
DpH because internal and external pH are equal. Above pH
7.6, internal pH becomes acidic relative to external pH (i.e., methylamine
is accumulated) although protons are being pumped out of the cell.
This behavior is due to the action of a sodium/proton antiporter (NhaA) that
exchanges one internal sodium ion for two external protons (i.e., it is
electrogenic), thereby acidifying the internal milieu, and the antiporter is
activated when the external pH rises above pH 7.6. Over the same pH range,
DY (as determined by TPP+
accumulation) rises from about –90 mV to about –110 mV. As a result of
these changes,
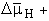 has
a maximum value of –210 mV at pH 5.0-5.5 and is composed of both
DpH and DY. At pH 7.6, has
a maximum value of –210 mV at pH 5.0-5.5 and is composed of both
DpH and DY. At pH 7.6,
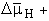 is
about –110 mV, and the sole component is DY.
Although not shown, above pH 7.6, is
about –110 mV, and the sole component is DY.
Although not shown, above pH 7.6,
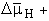 begins
to decrease because DpH has the
opposite polarity from DY. begins
to decrease because DpH has the
opposite polarity from DY.
Clearly, if accumulation of weak acids is coupled specifically to
DpH, dramatic accumulation should be observed at acidic pH, but
not at pH 7.6 and above. On the other hand, uniport of cations should
respond to pH in a manner similar to that shown for
DY, and substrates that are
transported by symport or antiport should respond in a manner similar to
that shown for
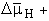 . .
|
