|
|
|
|
Bacteriophage HK97 capsid assembly is a premier model system for studying aspects of protein assembly mechanisms. Maturation of the head protein of HK97 includes interactions with chaperonins, assembly into interconvertible hexamers and pentamers, assembly into Prohead I together with portal and protease proteins, cleavage of the head protein and protease within the interior of the structure to create a new intermediate, Prohead II, expansion and interlinking of protein subunit chains, and the formation of novel covalent inter-peptide crosslinks. As a model system, the HK97 capsid has many advantages, 1) it is simple - only three genes are required to build it, 2) it is efficiently made in the laboratory using plasmid expression of cloned genes, 3) much of the pathway of its assembly and maturation are well understood, and 4) there is a large repertoire of structural information from both electron microscopy and x-ray crystallography.
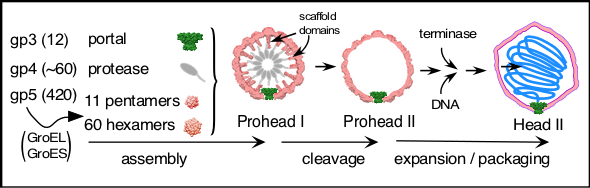
The HK97 head is assembled from two minor proteins gp3 (portal, 47 kDa), gp4 (protease, 25kDa), and the major head protein, gp5 (42kDa). The initial steps require the host chaperones GroEL & GroES and ATP. Prohead I is the first large intermediate and is converted to Prohead II when gp5 is cleaved from 42kDa to 31kDa under the control of gp4. The portion of gp5 that is lost is the scaffold domain (or delta domain) and lies inside the shell. The HK97 scaffold domain is functionally analogous to the separate scaffolding protein found in other capsids. Prohead II will mature and expand into a mature Head, usually at the time of DNA packaging. During expansion, reactive sites are created that cross-link gp5 subunits to adjacent subunits until all of the subunits are crosslinked in Head II.