|
|
|
|
| Project Objective: To determine the distance/angle dependence of CH-p interactions using a molecular torsion balance. |
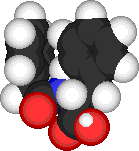 |
Significance: CH-p interactions have been proposed to be important inter-molecular forces, especially in peptides containing aromatic groups. An example at left shows a folded structure for N-phenylacetyl-L-Phe. (Fig. 1) |
| Figure 1. N-phenylacetyl-L-Phe |
| Progress: A molecular torsion balance was successfully used by our lab to measure CH-p interactions.(Ref 1,2 and Fig. 2) | 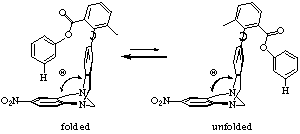 |
| Figure 2. Tröger's base derivative. |
| A similar torsion balance was synthesized to determine the distance/angle dependence of CH-p interactions. (Fig. 3) | 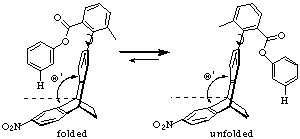 |
| Figure 3. Ethanoanthacene derivative. |
| Conclusion: The ethanoanthracene molecule prefers the unfolded conformation, but the Tröger's base molecule folds. Because the hinge angle is larger for the ethano-anthracene, the folded conformer fails to achieve favorable contacts. These results demonstrate how CH-p interactions are sensitive to subtle changes in distances. |
|
References: 1. S. Paliwal, S. Geib, and C. S. Wilcox "A Molecular Torsion Balance for Weak Molecular Forces. Effects of Tilted-T Edge-to-Face Aromatic Interactions on Conformational Selection and Solid State Structure." J. Amer. Chem. Soc. 1994 116, 4497-4498. 2. C. S. Wilcox, S. Paliwal, E. Kim "Measurements of Molecular Electrostatic Field Effects in Edge-to-Face Aromatic Interactions and CH-p Interactions with Implications for Protein Folding and Molecular Recognition." J. Amer. Chem. Soc., 1998 120, 11192-11193. |
[ Home | Related Sites | Members | Contact Us ]

