Growing environmental concern has demanded more selective, waste-free extractions to minimize the use of volatile organic solvents and simultaneously produce cleaner chromatograms with lower detection limits and higher accuracy. Extraction selectivity is greatly enhanced by incorporating artificial molecular receptors that interact non-covalently with a substrate in a highly selective and predictable manner (Valenta and Weber, 2006). Biological molecules are quite well known for their molecular recognition interactions and the first example of host/guest chemistry comes from nature. DNA complimentary base pairing, T-RNA binding, specificity in enzyme-substrate binding, neural transmitter and opiate reception at synapses, and protein based catalysis are just a few examples of naturally occurring molecular recognition interactions. The extent of molecular recognition is contingent on the size, shape and orientation of the molecules involved (see Figure 1.). Artificial receptors are not only useful for increasing selectivity and sensitivity in extractions and other selective separations methods, but molecular recognition it is also a great tool for creating novel sensors. Several families of artificial receptors are available today to create selective, reusable extraction/sensing material designed for specific analytes. We have been devoted to understanding and quantifying these molecular recognition interactions to create better, more accurate and faster analysis and develop novel sensing materials with endless applications.
 |
| Figure 1. Depiction of a molecular recognition interaction |
Extraction solvents influence both the solubility and partitioning of the substrate, receptor, and interfering matrix; they also influence the affinity of the receptor for the substrate. As a result, the free energy of an extraction can be optimized through careful selection of the solvent, which requires an in-depth understanding of the solvatochromatic parameters accompanying these extractions. We have performed a qualitative solvatochromatic analysis of poly- (vinyl chloride) plasticizers and their solutions in chloroform and applied it to better understanding the partitioning and molecular recognition of Phenobarbital (Valenta, et al, 1998). A similar solvatochromatic analysis was performed to predict the extraction purity and yield for a phenobarbital receptor-enhanced extraction and can be generalized to aid in optimal solvent selection and design of solvent and receptor for other extraction systems (Sun and Weber, 1998).
Much work has been carried out to pursue the development of a molecular recognition based Pb2+ sensor for environmental applications. Square wave voltammetry has been applied to determine the transport parameters of Pb2+ through a sensory gel to facilitate the understanding and optimization of a molecular recognition based Pb2+ sensor (Geary, et al, 2005). This square wave technology has also been applied to understanding the rate parameters of other metal ion-ligand complex systems involved in sensing, separations, and responsive materials (Geary and Weber, 2003). We have also investigated the kinetics of a crown ether-containing chromene (shown below) that photoreversibly binds to Pb2+ in the dark and dissociates upon irradiation with near-UV light. This technology has many promising applications for creating sensing and responsive materials and a complete understanding of the metal-crown kinetics is essential (Stauffer, et al, 1997).
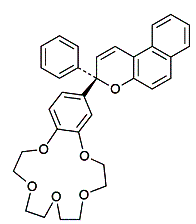 |
| 18-crown-6-containing Chromene |
The interest in photochromic compounds such as chromenes for their potential applications in optical switching, memory, and sensing has lead to study in which we found that fluoroalcohols are able to stabilize the merocyanine form of photochromic compounds through hydrogen bonding (Suzuki, et al, 1998). Photochromic molecules with functional groups that strongly bind to metal ions are appealing for applications as photoreversible sensors and preconcentrators for trace metal ion detection. We have synthesized a photochromic benzopyran that binds Zn2+ slowly in the dark but much more rapidly upon illumination with visible light, thus creating a novel photochromic metal chealtor that binds strongly to divalent metal ions (Stauffer and Weber, 1999). This led us to design a simple, reversible photochromic metal-ion chealator that could be easily and quantitatively anaylized. We were able to develop a chealator that binds to divalent metal ions in the dark, so that light could be used to control the release of metal ions rather than sequester them, and quantitatively analyze the photosensitivity of the complex (Stephens, et al, 2002).
The electrochemistry of charge transfer across the liquid/liquid interface has been used to understand the mechanisms of ion-selective electrodes and extractions processes. We have used cyclic voltammetry at the organic/aqueous interface to quantitatively study complex formation between a para-xylylenyl-bis(imino-imidazolinium) artificial receptor and a series of dicarboxyates (Shao, et al, 1998).
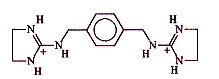 (1) |
(2) |
| Figure 2. (1) para-xylylenyl-bis(imidazolinium) artificial receptor and (2) a 1:1 complex of the para-xylylenyl-bis(imidazolinium) receptor with a glutarate substrate | |
Non Aqueous Affinity capillary electrophoresis (NAACE) is a relatively new approach for identifying and quantifying the extent of binding that gives the user more control over optimizing specific experimental parameters than traditional aqueous ACE. We applied this technique to examining the molecular interactions between the artificial molecular receptor 2 and a series of dicarboxylate anions. NAACE has also allowed us to effectively increase association constant between receptor and substrate by eliminating binding competition brought about from the need to use a carboxylate-containing buffer (Peddicord and Weber, 2002).
Our publications in last 10 years
Valenta, J. N.; Weber, S. G. “Molecular Recognition of Phenobarbital in Plasticizers: Equilibrium Investigations on the Solubility on the Barbiturate Artificial Receptor, and its Binding to Phenobarbital in Plasticizers”, J. Chromatogr. A 1996, 722, 47-57.
Wise, E. T.; Weber, S. G. “Reversibly Crosslinked Gels”, Polymeric Materials Encyclopedia, CRC Press, 1996.
Stauffer, M. T.; Knowles, D. B.; Brennan, C.; Funderburk, L.; Lin, F.-T.; Weber, S. G. “Optical Control over Pb2+ Binding to a Crown Ether-containing Chromene”, Chem. Commun. 1997, 287-288.
Valenta, J.; Sun, L.; Ren, L.; Weber, S. G. “Solvatochromic Study of Poly(vinylchloride) Plasticizers and their Solutions in Chloroform, and Application to Phenobarbital Partitioning and Molecular Recognition of Phenobarbital” Anal. Chem. 1997, 69, 3490-3495.
Shao, Y.; Linton, B.; Hamilton, A. D.; Weber, S. G. “Electrochemical Studies on Molecular Recognition of Anions: Complex Formation Between Xylylenyl Bis-iminoimidazolinium and Dicarboxylates in Nitrobenzene and Water”, Journal of Electroanalytical Chemistry, 1998, 441, 33-37.
Sun, L.; Weber, S. G. “Prediction of Molecular Recognition-Enhanced Phenobarbital Extraction Based on Solvatochromic Analysis”, Journal of Molecular Recognition 1998, 11, 28-31.
Suzuki, T.; Lin, F.-T.; Priyadashy, S.; Weber, S. G. “Stabilization of the Merocyanine Form of Photochromic Compounds in Fluoroalcohols is Due to a Hydrogen Bond”, Chem. Commun. 1998, 2685-2686.
Stauffer, M. T.; Weber, S. G. “Optical Control of Divalent Metal Ion Binding to a Photochromic Catechol: Photoreversal of Tightly Bound Zn2+, Anal. Chem. 1999, 71(6), 1146-1151.
Preigh, J. J.; Lin, F. T.; Ismail, K. Z.; Weber, S. G. “Bivalent metal ion dependent photochromism and photofluorochromism from a spiroquinoxazine,” Chem. Comm. 1999, 1, 105.
Stephens, M.R.; Geary, C. D.; Weber, S. G. “Kinetic analysis of a photosensitive chelator and its complex with Zn(II), Photochem. Photobio. 2002, 75(3), 211-220.
Peddicord, M.B.; Weber, S. G. “Nonaqueous affinity capillary electrophoresis (NAACE) investigation of primitive molecular recognition,” Electrophoresis 2002, 23(3), 432-436.
Geary, C. D., Weber, S. G., “Measurement of Association and Dissociation Rate Constants for Lead(II)/18-Crown-6 Using Square Wave Voltammetry at a Glassy Carbon Mercury Film Electrode,” Anal. Chem. 2003, 75(23), 6560-6565.
Geary, C. D., Zudans, I., Gopenenko, A. V., Asher, S. A., Weber, S. G., “Electrochemical investigation of Pb2+ binding and transport through a polymerized crystalline colloidal array hydrogel containing benzo-18-crown-6” Anal. Chem. 2005, 77, 185-192.
------------------------------------------------------------------------------------------------------------------------
The term ‘molecular recognition’ became popular in the early 1980s and implies a complementary “lock-and-key” type fit between (or within) molecules. The study of molecular recognition covers a set of phenomena that are controlled by specific noncovalent interactions.1 Such phenomena are crucial in biochemical systems, such as enzyme action, molecular transport, genetic information and processing, protein assembly, etc. In most cases, molecular recognition is defined as “host-guest chemistry”, implying a specific intermolecular interaction of a receptor molecule with a substrate one. For the recognition interaction to be effective, receptors are generally required to be compatible with the substrate in size, shape, and charge density. An additional requirement for receptors is precise alignment of multiple binding groups on the receptor with complementary regions on the substrate to provide both orientation and selective complexation of the substrate.2 Molecular recognition can also apply to intramolecular processes such as protein folding.3
Modern chemical research is motivated by the prospect that molecular recognition by design could lead to new technologies.1 Most studies are toward one of the following themes: elucidation of the role of noncovalent interactions, application of molecular recognition principles to practical goals, and extrapolation from biological examples.
In our group, molecular recognition has been applied to liquid-liquid extraction [Valenta, 1997], solid phase microextraction [Li, 1999; Zhang, 2003], and membrane transport [Zhang, 2002; Zhang, 2003] in achieving high selectivity.
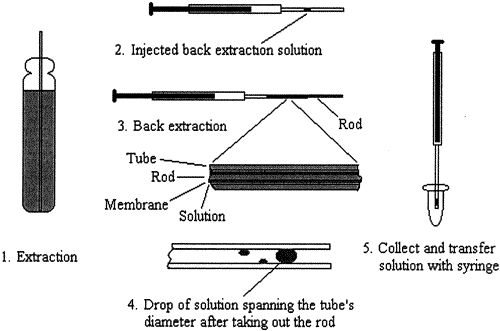
Solid phase extraction (SPE) is more time efficient and less labor intensive than conventional liquid-liquid extraction methods. Another advantage of solid phase extraction is the minimal solvent consumption and sample volume required for an effective separation, particularly in the case of solid phase microextraction (SPME) in which the extraction phase is just a small coated fiber or tip. Our group was among the first to couple SPME to CE [Li, 1997]. The SPME device is based on plasticized poly(vinyl chloride) (PVC) as an extraction solvent, which is coated on a primed steel rod (Figure 1).
 |
| Figure 1 |
An artificial receptor (Figure 2) has proven effective at improving the selectivity for barbiturates in SPME [Li, 1999]. The receptor is designed to dissolve in plasticized PVC. SPMEs using receptor-doped films are carried out as a function of receptor concentration. The effect of the concentration of the receptor on extraction yield is considerable for barbiturates that have significant binding to the receptor but negligible for very similar molecules that do not bind to the receptor strongly. Thus, it is the receptor's recognition ability, not its generic ability as an H-bonding co-solvent, that is important.
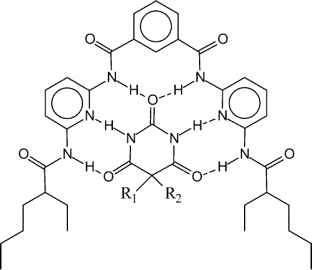 |
| Figure 2 |
It would be beneficial to have selective extractions for any given organic species; however, such receptors do not exist. In order to find good receptors from libraries of potential receptors, a screening method has been proposed based on membrane transport [Zhang, 2002]. This work examines whether we can establish a sufficient free energy gradient for a good receptor to move to a predetermined place in space. In solutions bathing the two sides of a plasticized PVC membrane containing the receptor, a difference in the solute concentration creates a spatial concentration gradient of the solute in the membrane at steady state (Figure 3). This causes the receptor's chemical potential to vary across the membrane. Upon binding to the solute, the receptor undergoes a local activity drop, which decreases its free energy. This process produces a flux of receptor to accumulate at place where there is a high solute concentration. The solute gradient is completely ignored by the receptor.
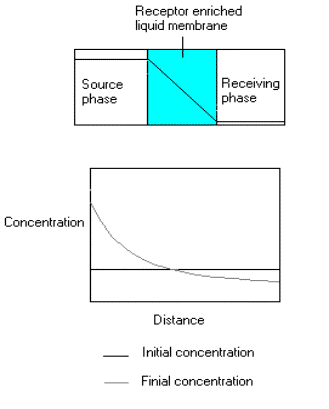 |
| Figure 3. Concentration gradient of the solute across membrane (upper panel) and concentration gradient of the receptor in the membrane (lower panel) |
In a further study of molecular recognition in SPME, we have found that the polymer concentration in the plasticized PVC membrane plays an important role in the selectivity of the extraction [Zhang, 2003]. Partition coefficients for phenobarbital decrease as PVC ratio increases, while the formation constants for the complex of the solute with its receptor increase. The increase in polymer concentration brings about a decrease in hydrogen-bonding basicity and an increase in dipolarity and cohesive energy density. The values of the solvatochromic parameters determined at various compositions are highly correlated; thus, it is impossible to calculate how much each factor contributes to the changes associated with partition and complex formation. The solvatochromic "polarizability correction factor" has been determined to be 0 for PVC. SPME experiments were performed at 30%, 40%, and 50% (w/w) PVC. It was found that, as polymer concentration increases, selectivity for barbiturate extraction over other cyclic imides increases in the presence of barbiturate receptor and decreases without it.
 |
|
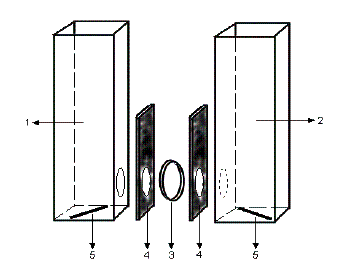
Figure 4. Schematic diagram of
the transport apparatus. 1. cuvette for the source phase; 2. cuvette for the receiving phase; 3. film; 4. Vition rubber; 5. stirring bars. |
Fluorous media have great potential for selective extraction (e.g., as
applied to organic synthesis). Fluorous polymer films would have many
applications in fluorous separations. We have investigated Teflon AF 2400,
a stable and permeable fluorous polymer, as a transport/extraction medium
for solutes for the first time [Zhao, 2004; Zhao 2005]. Rigid aromatic
solutes are transported (Figure 4) according to size. The films show a
high selectivity for fluorinated solutes in comparison to the
hydrogen-containing control. Along with detailed measurement of the
partitioning and diffusion of benzene in the films, we have showed that
the Teflon AF film can be plasticized by solvents to which it is exposed,
namely chloroform. Krytox FSH, a carboxylic acid terminated
perfluoropolyether, also plasticizes films. This carboxylic acid is
capable of molecular recognition in the film. The noncovalent association
between Krytox FSH (0.13 M in the film) and 3-hydroxypyridine increases
the distribution ratio of the polar solute into the film 41 times. In
comparison, the partition ratio into a fluorocarbon solvent (FC-72)
increases 15000 times under the same conditions, indicating that the films
of Teflon AF 2400 are not as fluorous as the FC-72 liquid.
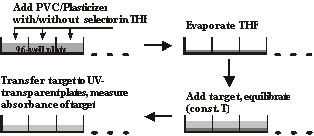
The most widely used technique for chiral separations at the analytical and semipreparative scale is liquid chromatography (LC) on chiral stationary phases (CSPs). The desire for more generality, better selectivity, more robustness and predictability drives the search for new CSPs. We are currently collaborating with Professor Peter Wipf in the organic division to develop new CSPs using combinatorial chemistry. Libraries of peptide mimics have potential applications as CSPs. A high-throughput screening protocol has been developed for evaluation of their enantioselectivity [Chen, 2006]. It is modeled after the protocol for biological screening of candidate drugs from chemical libraries. The procedure works based on target distribution between an aqueous phase and an organic phase (plasticized PVC). Screening for noncovalent intermolecular association between target and candidate selectors is carried out by partitioning experiments with and without the presence of the chiral selector candidates in plasticized PVC films. The partition ratio measurement uses 96-well plates for high throughput (Figure 5). The advantage of this method is that it does not require the covalent attachment of analyte or selector, and the required amount of the potential chiral selector (~100 µg) is small compared to other screening methods (15-50 mg).
 |
| Figure 5 |
References