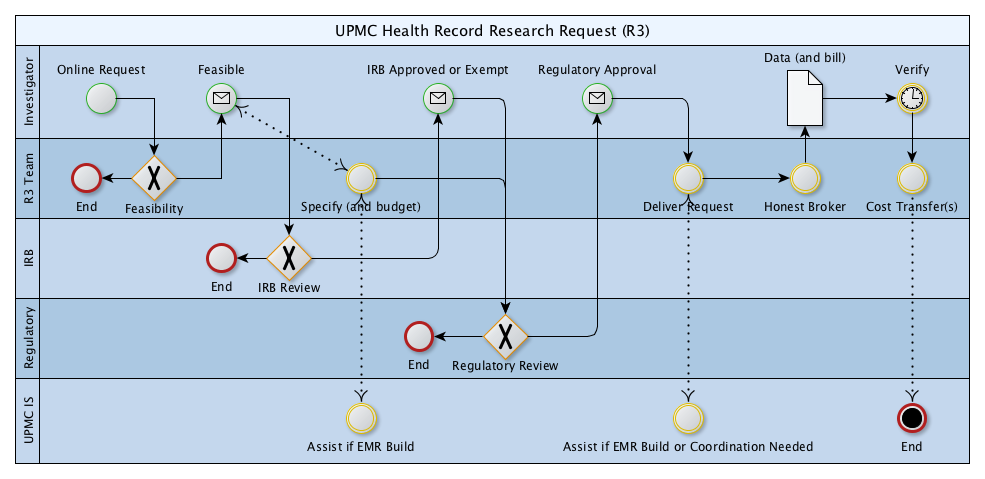
Initial contact with R3 is via online form accessible only by Pitt credentials.
This form establishes primary contact, principal investigator, and high level information regarding the project
as well as datetime stamp of the initial contact and assigns an identifier to the workflow.
In response to each online request, the R3 Team has at least one brief communication with the investigator
to confirm the research goals and to identify more specifically the health record research request.
If the project appears infeasible, the workflow ends
by informing the investigator of why
and paths forward may be suggested
(e.g. if the data does not exist, if the data is not
accessible for research for clear regulatory reason).
The investigator leads the IRB process with support as appropriate from the R3 Team.
The R3 Team can only deliver records with IRB approval or exemption or research deemed not human subjects.
Patient count only requests may be streamlined (they may be deemed not human subjects research
and may complete the entire R3 workflow at one sitting).
If none of IRB Approval, IRB Exemption, or deemed not human subjects can be achieved, then the process ends.
If the project appears feasible, the investigator is notified.
At this point two parallel workflows are initiated.
The investigator begins the IRB Review process while
the R3 Team specifies the precise R3 to be delivered.
Assist if EMR Build
The R3 Team leads the process of documenting and costing the precise deliverable.
This will typically involve iterative conversation with the investigator team
who may use the information in IRB documents.
This activity may involve UPMC ISD (particularly if new data sources or EMR build is required).
IRB Approved or Exempt
Regulatory Review
End
Regulatory Approval
Deliver Request
Authorization and Prioritization for use of EMR data and workflows
is via UPMC Chief Medical Information Officer.
Data is delivered via Pitt Box account in concert with documentation and invoice.
Honest brokerage (linking, de-identification and
provisioning of protected health information)
is under honest broker certification held by the
Chief Research Informatics Officer including
honest brokers in the Department of Biomedical Informatics.
The investigator verifies receipt of data and acknowledges cost transfers will occur in a timely fashion.
The CRIO will manage cost transfers in account at DBMI.
The R3 process is complete when cost transfers are
complete among the investigator account and the DBMI
charge-back account (and UPMC information services
accounts if applicable).