Anyone who has ever made a cup of coffee or hot tea has performed an extraction. Extraction is a fundamental technique used to isolate one compound from a mixture. Becoming familiar with its theory and correct use are essential to successful completion of many organic experiments. The three most common types of extractions are: liquid/liquid, liquid/solid, and acid/base (also known as a chemically active extraction). The coffee and tea examples are both of the liquid/solid type in which a compound (caffeine) is isolated from a solid mixture by using a liquid extraction solvent (water).
LIQUID/LIQUID EXTRACTION
A liquid/liquid extraction involves two immiscible liquids. Immiscible liquids do not dissolve in each other; they form layers when placed in the same glassware. Immiscibility is a result of two liquids having different polarity. The most common pair of extraction solvents used is diethyl ether (often referred to as simply 'ether') and water. Polarity is a relative term - ether is considered nonpolar and water polar. The fact that two phases are observed upon adding one to the other is a consequence of their different polarities. The location (either the top or bottom layer) of an extraction solvent is determined by density. The density of ether is 0.713 g/cm3 and the density of H2O is 1.0 g/cm3; therefore, ether is always the top phase when the extraction solvent pair is ether and water.
HOW TO PERFORM
Extractions are performed in a separatory funnel . When two immiscible liquids are placed in the separatory funnel two phases are observed as discussed above. As mentioned before, the fundamental reason for carrying out an extraction is to isolate a compound from a mixture. For example, consider a mixture consisting of 2 polar compounds and 1 nonpolar compound. After extraction with the solvent pair of ether and water, the 2 polar compounds would be found in the aqueous layer (a polar solvent dissolves a polar solute) and the nonpolar compound would be found in the nonpolar phase (ether). Note: the phase consisting of H2O is called the aqueous phase.
As a result, the nonpolar compound is isolated from the mixture. (See the extraction scheme below.)
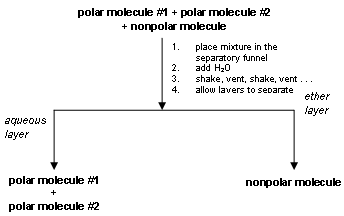
'Like dissolves like' is the general phrase used as a reminder that compounds of similar polarity are miscible. The idea that a compound dissolves more or less readily in a solvent can be quantitated by the distribution coefficient:
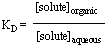
which says there exists a ratio of the concentration of the solute between the two phases given the solvent pair and temperature. This coefficient is discussed further in recitation.



