Microreactor Design & Applications
1. Motivation
In recent years, miniaturized total analytical systems (μTAS) have been an area of great activity.1, 2 Although many μTAS systems involve separations, there are many that do not. The non-separatory μTAS consist of reagents, microchannels and on- or offline detection system. These systems are capable of integrating all stages of a complete analysis, including sampling, sample pretreatment, chemical reaction, separation, detection, and data processing in an highly automated and efficient manner.3 Many researchers were inspired by the merit of mTAS and have begun to focus on miniaturizing synthetic chemistry into microscale reactors.4
Microreactors are advantageous to synthetic chemistry because of decreased dimensions of the reaction environment that result in better heat transfer efficiency, shorter reagent mixing time and less chemical consumption than the conventional scale chemistry. The chemical reaction occurs in a flow stream inside the microreactor where laminar flow dominates fluid transport5 enabling more precise control of reaction parameters such as concentration gradients, temperature, and pH. The minimal reagent use, exellent reaction control and containment of microreactors make it much safer to perform dangerous or poisonous reactions.6
Microreactors have been applied to typical organic synthesis, catalyst discovery and combinatorial chemistry. For example, in a novel application, Comer and Organ have mated a microreactor with microwave heating to give the reaction mixture 4 minutes of exposure to radiation.7 Catalysts for following systems and reactions have been investigated: catalysts for gas/liquid systems,8 polymerization, Kumada coupling,9 Baeyer-Villager oxidation,10 expoxidation.11 Microreactors have also been applied to multi-step reactions and parallel screening.12
2. Microreactor design13
Although applications of the microreactor are growing rapidly, most of them are limited to fast reactions. Investigators borrow the setup of µTAS systems and apply them to organic reactions and the low capacity of chip devices make this one-sample-at-a-time approach only applicable to relatively rapid reactions. Few reactors have been developed for slow reactions limiting the application and demand for such systems.
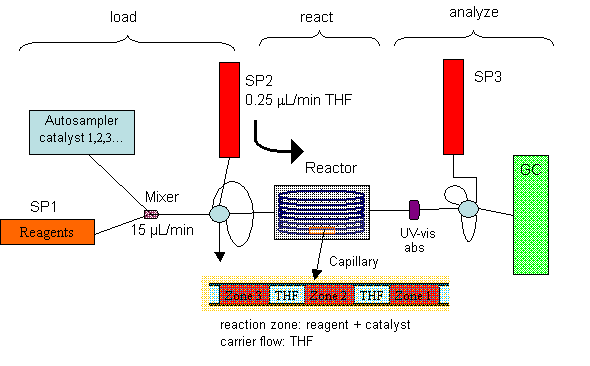
The goal of our research is to develop a novel microreactor system specifically designed for high-throughput screening of slow organic reactions accelerated by homogeneous catalysts. The design of the microreactor is shown in Scheme 1. We integrate standard HPLC apparatus (autosampler, pump), fused silica capillaries and GC to build a computer-controlled reactor system in which separate zones of reactants and catalysts can be combined and loaded serially into the reactor capillary, reacted in parallel and ejected serially for online GC analysis. Offline analysis following the sample collection is also possible.
|
|
|
Scheme 1. A schematic diagram of the microreactor system. |
The loading section consists of a syringe pump (SP1) for the reagents and another for the driving flow (SP2). Catalysts are combined with reagents at equal flow rates into the loop of a 6-port microinjector by an autosampler. When the auto-sampler has completed its cycle, the loop’s contents are pushed into the reactor. The reactor can be heated. The detection section includes a UV-Vis absorbance detector for locating the reaction zones. Under computer control, the zones are alternately pushed into one loop in the double-loop, 10-port microinjector by SP2, and then injected into the capillary GC by SP3. The injection by SP3 initiates the GC program for quantitative analysis of the reaction products.
3. Microreactor applications
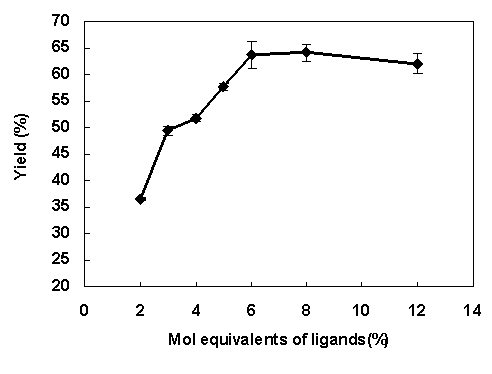
We first evaluate the performance of the microreactor by investigating catalysts for the Stille reaction, which is one of the most important reactions leading to the formation of carbon-carbon bonds.14 The reaction is catalyzed by a variety of palladium catalysts complexed with neutral ligands. The screening results are in a good agreement with literature. For example, PdCl2(CH3CN)2 (2 mol%) + AsPh3 (6 mol%) is the optimal catalyst for the Stille reaction in our tests (Figure 1).13
|
|
|
Figure 1. Yields of Stille reaction with different mole equivalents of AsPh3 and 2 mol% equiv PdCl2(CH3CN)2. |
The current work concentrates on applying this reactor to screen peptides/mimics for chiral catalysis in asymmetric aldol reactions.15 The aldol reaction is believed to be one of most powerful carbon-carbon bond forming reactions. Through the chiral catalysis of peptides, this reaction gives high yields and excellent stereoselectivity under mild conditions.16 Our aim is to use the microreactor to screen a library of active peptides for aldol reaction – a task significant for both synthetic and combinatorial chemistry. Now, we are focusing on the optimization of reaction yields and enantioselectivity of β–hydroxyketone. Future work will include reactor optimization and library development of organic reactions.
The ultimate goal of our research is to develop a highly automated and high-throughput microreactor system in support of synthetic chemistry. This would have an immediate impact on biology through the development of pharmaceuticals.
Reference
-
Brivio, M.; Fokkens, R. H.; Verboom, W.; Reinhoudt, D. N.; Tas, N. R.; Goedbloed, M.; van den Berg, A., Integrated Microfluidic System Enabling (Bio)chemical Reactions with On-Line MALDI-TOF Mass Spectrometry. Analytical Chemistry 2002, 74, (16), 3972-3976.
-
Ramsey, J. M., The burgeoning power of the shrinking laboratory. Nature Biotechnology 1999, 17, (11), 1061-1062.
-
Jakeway, S. C.; de Mello, A. J.; Russell, E. L., Miniaturized total analysis systems for biological analysis. Fresenius' Journal of Analytical Chemistry 2000, 366, (6-7), 525-539.
-
Haswell, S. J., Miniaturization - What's in it for chemistry? Micro Total Analysis Systems 2001, Proceedings mTAS 2001 Symposium, 5th, Monterey, CA, United States, Oct. 21-25, 2001 2001, 637-639.
-
Jas, G.; Kirschning, A., Continuous flow techniques in organic synthesis. Chemistry--A European Journal 2003, 9, (23), 5708-5723.
-
Jaehnisch, K.; Hessel, V.; Loewe, H.; Baerns, M., Chemistry in microstructured reactors. Angewandte Chemie, International Edition 2004, 43, (4), 406-446.
-
Comer, E.; Organ, M. G., A Microreactor for Microwave-Assisted Capillary (Continuous Flow) Organic Synthesis. Journal of the American Chemical Society 2005, 127, (22), 8160-8167.
-
De Bellefon, C.; Tanchoux, N.; Caravieilhes, S.; Grenoullet, P.; Hessel, V., Microreactors for dynamic, high-throughput screening of fluid/liquid molecular catalysis. Angewandte Chemie, International Edition 2000, 39, (19), 3442-3445.
-
Haswell, S. J.; O'Sullivan, B.; Styring, P., Kumada-Corriu reactions in a pressure-driven microflow reactor. Lab on a Chip 2001, 1, (2), 164-166.
-
Mikami, K.; Islam, M. N.; Yamanaka, M.; Itoh, Y.; Shinoda, M.; Kudo, K., Nanoflow system for perfect regiocontrol in the Baeyer-Villiger oxidation by aqueous hydrogen peroxide using lowest concentration of a fluorous lanthanide catalyst. Tetrahedron Letters 2004, 45, (18), 3681-3683.
-
Wan, Y. S. S.; Chau, J. L. H.; Yeung, K. L.; Gavriilidis, A., 1-Pentene epoxidation in catalytic microfabricated reactors. Journal of Catalysis 2004, 223, (2), 241-249.
-
Cullen, C. J.; Wootton, R. C. R.; de Mello, A. J., Microfluidic systems for high-throughput and combinatorial chemistry. Current Opinion in Drug Discovery & Development 2004, 7, (6), 798-806.
-
Shi, G.; Hong, F.; Liang, Q.; Fang, H.; Nelson, S.; Weber, S. G., Capillary-Based, Serial-Loading, Parallel Microreactor for Catalyst Screening. Analytical Chemistry 2006, 78, (6), 1972-1979.
-
Farina, V.; Krishnan, B., Large rate accelerations in the stille reaction with tri-2-furylphosphine and triphenylarsine as palladium ligands: mechanistic and synthetic implications. Journal of the American Chemical Society 1991, 113, (25), 9585-95.
-
Krattiger, P.; Kovasy, R.; Revell Jefferson, D.; Ivan, S.; Wennemers, H., Increased structural complexity leads to higher activity: peptides as efficient and versatile catalysts for asymmetric aldol reactions. Organic letters 2005, 7, (6), 1101-3.
-
Machajewski, T. D.; Wong, C.-H.; Lerner, R. A., The catalytic asymmetric aldol reaction. Angewandte Chemie, International Edition 2000, 39, (8), 1352-1374.