Applications of Electroporation
Electroporation, or electropermeablization, of cells and liposomes has been observed and applied for many decades.1-8 An electric field created in a conducting solution causes the membranes of suspended cells to become permeable. Under suitable conditions, the pores formed in the membrane are transient, and most of the cells subjected to the electric field will survive. Electroporation is therefore a powerful method for introducing cell-impermeant species into living cells. The alternative is to modify chemically cell-impermeant species so that they passively diffuse through the membrane. While this suffices in some applications, for example the introduction of polar Ca2+-sensitive dyes as nonpolar esters,9 for many biologically relevant species, such as DNA, RNA, ions, particles, proteins, libraries of compounds, it is inconvenient or impossible. In addition, electric fields can also induce fusion of cells and liposomes. Of course, electroporation on cell suspensions (which will be referred to as “bulk electroporation”) gives rise to a statistical distribution of cellular states.10, 11 The phenomena and parameters described should be considered averages.
In medical and biological research, attention is turning to the use of single or small populations of cells in many different settings and experiments. Examples include, functional cell-based drug screening, stem cell research, flow cytometry and many more. Microfabrication technologies for developing chip-based devices for cell sorting, manipulation and characterization are likely to be extremely successful. In addition, modern analytical chemistry can provide quantitative data on biological and biochemical phenomena, sometimes at the single-cell level. The hypothesis is that a study of many individual single cells will lead to a more profound understanding than the study of the same cells as an ensemble. A seemingly small but critical modification to this hypothesis is necessary: single cells really need to be studied in situ. While there is no doubt that studies on single cells from cell lines or, for example, Xenopous oocytes, will move us forward, we really need to know how single cells operate within a population of cells. This means that single cells must be addressed without irreversible damage.
Methods available today for internalization of charged or polar bioactive compounds are either different types of microinjection methods or methods where the cell membrane is transiently porated. Microinjection methods are most common, where a thin glass needle is inserted into the cell using mechanical force (stab injection) and by a small amount of fluid is injected into the cytoplasm by controlled pressure,12 The pressure is usually generated by pressurized-air systems. There are also reports of pressure control by means of thermal manipulation, where an injection needle is first back-filled with the material to be introduced followed by a thermosensitive expansion medium, such as galinstan. The main drawback of microinjection is that small tip diameters (typically a few hundred nm) are needed for successful penetration and this limits the size of material to be introduced or withdrawn from a cell. In order to prevent clogging, the material must be smaller than the tip diameter. Materials such as large polymers could also be mechanically damaged from the shearing forces created at small tips. In microinjection methods there is also the possibility of cellular damage from mechanical stress, which is unpredictable and frequently high. Also, microinjection methods add both pressure and volume to a cell which is becomes more difficult to control as the size of the cell gets smaller. This is predicted to create a large problem in organelle injections where container sizes can be on the order of a few hundred nanometers in diameter and carry volumes of 10-18 L or less. It has been demonstrated that stab microinjection methods are extremely difficult to use with liposomes because they lack internal structure. In contrast, electroporation allows impermeable substances to be introduced in the cytoplasm irrespective of the container size (containers less than a micrometer in diameter work fine), since it does not add any extra volume/ pressure in the container, and has proven to work well with liposomes. Electroporation is a fast and robust method and typically results in an extremely high yield of viable cells.
Single cell electroporation is key in understanding biology at this level. It is – literally – like having a window into an operating cell. True single-cell electroporation platforms12 and somewhat lower resolution setups exist.13 It is a recent development, but there is no question that this experiment ‘works’ at a mechanical level. So, what is left to be done? Plenty.
We aim to make these important tools predictable and quantitative, give them more spatial resolution, and demonstrate single cell analysis in two ways: introducing reporter molecules followed by microscopy and sampling the contents of a cell followed by microanalysis. No other method that we can envision will allow analysis and/or control over single cells in a population of cells with such minimal damage and high precision and accuracy.
More Technical Background on Electroporation
The process of electroporation can be divided into two steps: pore formation and pore transformation. The membrane is permeated only when the transmembrane potential (TMP) is larger than a certain threshold value. Exactly how this affects transport across the membrane is contradictory in the literature. Some reports suggest that value of TMP has no affect on the degree of permeabilization14 while other models assume that transport of molecules across membrane is a function of TMP.15 However, all authors agree that the electric field around the cell determines which portions of the membrane become permeable.14, 15 The duration of the electric stimulus controls pore growth and, as a reslult, the degree of membrane permeation. Other factors, such as buffer composition and temperature, also determine pore dynamics. These effects are often observed in the resealing phase after an electric pulse has been applied.
The electric field
If a spherical cell is placed in a steady field, it will feel a
potential difference across its membrane16 of approximately
![]()
where
![]() is the field in the solution,
rcell is the cell radius, and
is the field in the solution,
rcell is the cell radius, and
![]() is the angle shown in Fig. 1.
is the angle shown in Fig. 1.
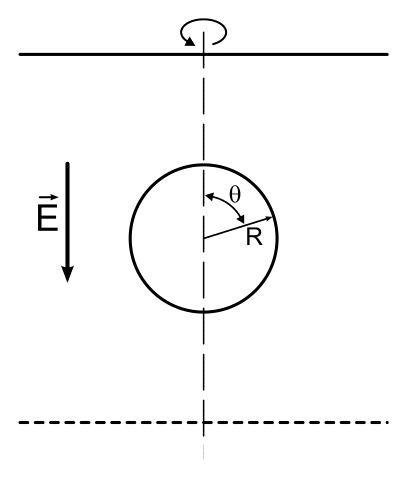 |
| Figure 1. A schematic representation of a cell in a suspension between two large parallel electrodes. The rotation sysmetry axis going through the cell is shown. |
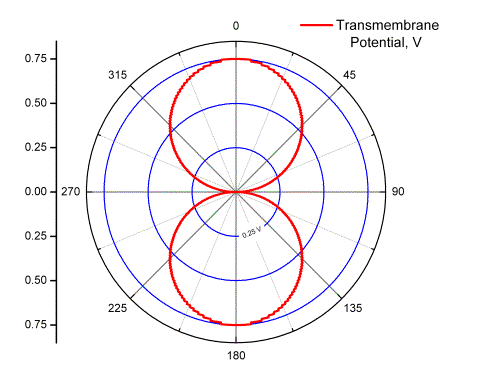
This simple result arises from assuming that the membrane resistance is much higher than the resistance within the cytosol, the field is homogeneous, and that the cell is a sphere. As shown in Figure 2, the TMP is largest on the poles of the cell facing electrodes. The first blue circle in the figure marks critical value of TMPc = 0.25V. At this field strength a large portion of the membrane is above the critical value, leaving only the sides of the cell non-permeable because TMP < TMPc. This is schematically illustrated in Figure 3.
 |
|
Figure 2.
|TMP| vs.
|
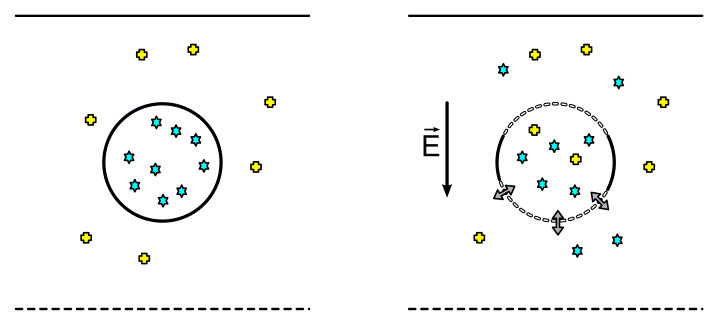 |
| Figure 3. Cell before(left) and after(right) electroporation. Permeated membrane is shown with stylized line and impremeable memebrane with solid line. |
The normal assumption in bulk electroporation is that the field in solution is the applied voltage (hundreds to a thousand volts) divided by the interelectrode spacing (0.1-1 cm). This assumption has recently been investigated by Pliquett et al.17 They showed that this can be true, but that the magnitude of the effective field may depend on the buffer type. This assumption is fairly good.
The situation is completely different for single-cell electroporation. In microelectrode- and capillary-based electroporation, the field is not homogeneous, it is higher near the source of the field. For example, the magnitude of the electric field extending into solution along the axis of symmetry of the capillary is given by:18
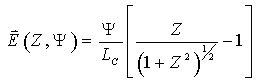
where
![]() is the electric field,
is the electric field,
![]() is the applied potential, Lc
is the column length , and Z is the dimensionless distance from
the tip of the capillary, z/a (where z is the distance
from the capillary tip and a is the capillary lumen radius). For
a microelectrode an additional complication arises - only a very small
fraction of the applied voltage ends up as the field in solution. If a
few (rather than a few hundred to one thousand) volts are applied
between a pair of electrodes, then only some a fraction of that voltage
will appear across the solution. We are currently developing theoretical
end experimental methods for assessment of the field strength near
microelectrodes. Microelectrode preparation methods are also refined to
enable preparation of various electrode tip shapes.
is the applied potential, Lc
is the column length , and Z is the dimensionless distance from
the tip of the capillary, z/a (where z is the distance
from the capillary tip and a is the capillary lumen radius). For
a microelectrode an additional complication arises - only a very small
fraction of the applied voltage ends up as the field in solution. If a
few (rather than a few hundred to one thousand) volts are applied
between a pair of electrodes, then only some a fraction of that voltage
will appear across the solution. We are currently developing theoretical
end experimental methods for assessment of the field strength near
microelectrodes. Microelectrode preparation methods are also refined to
enable preparation of various electrode tip shapes.
Model of pore formation induced by electric fields
Pores in the membrane are treated as chemical entities with an energy based on equilibrium considerations.3, 4 Pore formation and disappearance is modeled as a reactive diffusion problem in which there are chemical reactions for formation and disappearance, the rates of which depend on the pore radius. Pore growth is governed by a diffusion equation, in which thermal forces act on the pore radius and the pore radii respond to chemical potential gradients. The pore reaction can be written as below in which C is closed, O is open and hydrophobic, and O* is open and hydrophilic. When a hydrophobic pore reaches a radius of ~5 nm it will convert into a hydrophilic pore with the polar headgroups facing into the pore. If the hydrophilic pore enlarges beyond a critical radius, it will not reseal and eventually the cell will die.
![]()
The pore energy has several terms. The simplest model has terms for surface tension and its one-dimensional analog, line tension, and an electrostatic energy term for the replacement of lipid by water in the pore. From quantitative estimates to various parameters experimental results can be rationalized in these terms. Modeling can be used to determine pulse conditions that will or will not lead to irreversible cell damage and cell death. However, there is generally some distribution of cellular responses to a particular set of experimental conditions which complicates the interpretation.10, 11 Nonetheless, some observations are fairly robust.
Current understanding of bulk electroporation
- The transmembrane potential required for pore formation is estimated to be on the order of 0.2-1.5V, much higher than the natural transmembrane potential.15, 19, 20
- There is an inverse relationship between the required pulse time and applied pulse voltage in model systems (BLMs, liposomes).21 Similar observations on cells have been made: The applied field required for dye penetration decreases 4-fold (4 kV/cm to 1 kV/cm) on increasing pulse width from low ms range to 15 s.22
- Cell deformation accompanies the application of the field and the extent can be controlled through pulse characteristics and solution conductance.23, 24
- The conductance of the external medium has a significant effect on the observed results. Low conductance favors deformation.3
- Mechanical deformation of cells leads to an increase in lateral tension. As a result lower field strengths are required for permeabilization. The translocation yield (efficiency of extracellular solutes becoming intracellular solutes) is much higher for low molecular weight species than for large ones.
- Many pores are formed in a typical electroporation experiment. The fraction of membrane area covered by pores is estimated25 to be 0.1 to 0.01%
References
- Zimmermann, U.; Pilwat, G.; Riemann, F. Biophysical Journal 1974, 14, 881-899.
- Sale, A. J. H.; Hamilton, W. A. Biochimica et Biophysica Acta 1968, 163, 37-43.
- Neumann, E.; Sowers, A. E.; Jordan, C. A., Eds. Electroporation and electrofusion in cell biology; Plenum: New York, 1989.
- Chang, D. C. In Guide to Electroporation and Electrofusion; Saunders, J. A., Chassy, B. M., Sowers, A. E., Eds.; Academic Press, Inc., 1992, pp 9-27.
- Weaver, J. C.; Chizmadzhev, Y. A. Bioelectrochemistry and Bioenergetics 1996, 41, 135-160.
- Gass, G. V.; Chernomordik, L. V. Biochimica et biophysica acta 1990, 1023, 1-11.
- Joshi, R. P.; Schoenbach, K. H. Physical Review E 2000, 62, 1025-1033.
- Zimmermann, U.; Friedrich, U.; Mussauer, H.; Gessner, P.; Hamel, K.; Sukhorukov, V. IEEE Transactions on Plasma Science 2000, 28, 72-82.
- Johnson, I. Histochem. J. 1998, 30, 123-140.
- Gift, E. A.; Weaver, J. C. Biochimica et Biophysica Acta, Biomembranes 1995, 1234, 52-62.
- Bliss, J. G.; Harrison, G. I.; Mourant, J. R.; Powell, K. T.; Weaver, J. C. Bioelectrochemistry and Bioenergetics 1988, 20, 57-71.
- Haas, K.; Sin, W.-C.; Javaherian, A.; Li, Z.; Cline, H. T. Neuron 2001, 29, 583-591.
- Teruel, M. N.; Blanpied, T. A.; Shen, K.; Augustine, G. J.; Meyer, T. J. Neurosci. Meth. 1999, 93, 37-48.
- Rols, M. P.; Teissie, J. Biophysical Journal 1990, 58, 1089-1098.
- Puc, M.; Kotnik, T.; Mir, L. M.; Miklavcic, D. Bioelectrochemistry 2003, 60, 1-10.
- Kotnik, T.; Bobanovic, F.; Miklavcic, D. Bioelectrochemistry and Bioenergetics 1997, 43, 285-291.
- Pliquett, U.; Weaver, J. C. Bioelectrochemistry and Bioenergetics 1996, 39, 1-12.
- Nolkrantz, K.; Farre, C.; Brederlau, A.; Karlsson, R. I. D.; Brennan, C.; Eriksson, P. S.; Weber, S. G.; Sandberg, M.; Orwar, O. Analytical Chemistry 2001, 73, 4469-4477.
- Weaver, J. C. J. Cell. Biochem. 1993, 51, 426-435.
- Teissie, J.; Eynard, N.; Vernhes, M. C.; Benichou, A.; Ganeva, V.; Galutzov, B.; Cabanes, P. A. Bioelectrochemistry (Amsterdam, Netherlands) 2002, 55, 107-112.
- Muraji, M.; Taniguchi, H.; Tatebe, W.; Berg, H. Bioelectrochem. Bioenerg. 1999, 48, 485-488.
- Chang, D. C. In Guide to Electroporation and Electrofusion; Chang, D. C., Chassy, B. M., Saunders, J. A., Sowers, A. E., Eds.; Academic Press, Inc.: San Diego, 1992.
- Kinosita, J., K.; Hibino, M.; Itoh, H.; Shigemori, M.; Hirano, K.; Kirino, Y.; Hayakawa, T. In Guide to Electroporation and Electrofusion; Chang, D. C., Chassy, B. M., Saunders, J. A., Sowers, A. E., Eds.; Academic Press, Inc.: San Diego, 1992, pp 29-47.
- O'Neill, R. J.; Tung, L. Biophys. J. 1991, 59, 1028-1039.
- Hibino, M.; Shigemori, M.; Itoh, H.; Nagayama, K.; Kinosita, K., Jr. Biophysical journal 1991, 59, 209-220.