 |
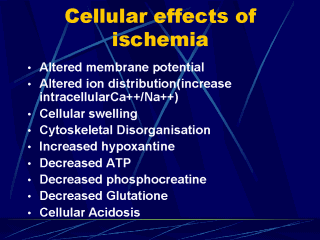
Prolonged
ischemia results in a variety of cellular metabolic and ultrastructural changes. Ischemia
induced decreases in cellular oxidative phosphorylation results in a failure to
resynthesize energy-rich phosphates, including adenosine 5'-triphosphate (ATP) and
phosphocreatine. Membrane ATP-dependent ionic pump function is thus altered, favoring the
entry of calcium, sodium, and water into the cell. Furthermore, adenine nucleotide
catabolism during ischemia results in the intracellular accumulation of hypoxanthine,
which is subsequently converted into toxic reactive oxygen species (ROS) upon the
reintroduction of molecular oxygen see below). Within the endothelium, ischemia promotes
expression of certain proinflammatory gene products (e.g., leukocyte adhesion
molecules, cytokines) and bioactive agents (e.g., endothelin, thromboxane A2),
while repressing other “protective” gene products (e.g., constitutive nitric
oxide synthase, thrombomodulin) and bioactive agents (e.g., prostacyclin, nitric
oxide). Thus, ischemia induces a proinflammatory state that increases tissue vulnerability
to further injury on reperfusion. |
