 |
The
Pravastatin Inflammation/CRP Evaluation (PRINCE) was a community-based prospective trial
that included both a primary-prevention component, in which 1702 men and women without
prior history of cardiovascular disease were randomized to pravastatin 40 mg/d or placebo,
and a secondary-prevention component, in which 1182 men and women with a history of
myocardial infarction, stroke, or arterial revascularization were assigned open-label
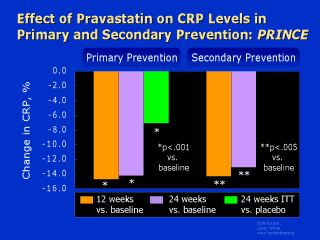
pravastatin 40 mg/d. In pravastatin patients with adequate follow-up data, CRP was
significantly reduced from baseline by 14.7% (0.02 mg/dL) in primary prevention and by
14.3% (0.02 mg/dL) in secondary prevention at 12 weeks, and by 14.2% and 13.1%
respectively at 24 weeks. On assumption of "no change" in primary-prevention
patients with inadequate data (n=311), CRP reduction at 24 weeks was 7.1% (0.012 mg/dL;
p<.001) with pravastatin vs. placebo. In correlational analyses of pravastatin
patients, there were minimal associations between 24-week change in CRP and changes in
lipid levels. In linear regression analyses, the only significant predictors of change in
CRP on a log scale were pravastatin and baseline CRP. Reference:
Albert MA, Danielson E, Rifai N, Ridker PM, for the PRINCE Investigators. Effect of statin
therapy on C-reactive protein levels: the Pravastatin Inflammation/CRP Evaluation
(PRINCE): a randomized trial and cohort study. JAMA 2001;286:64-70.
Web
site |
