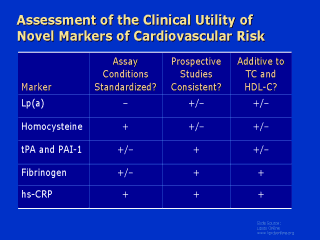
 |
Several years
ago, we began a process of evaluating novel risk factors for cardiovascular disease and
identified three critical criteria. First, is there an assay that has standardized
conditions, so that if we measure it with one clinical chemistry setting, we get the same
value as we do in another? This has been a major issue for lipoprotein(a), a unique marker
of atherothrombosis that unfortunately can be very difficult to measure and still has not
been standardized at a clinical level. The second criterion is the requirement for a
consistent series of prospective epidemiologic studies. Prospective studies are those that
ascertain the abnormality of interest before the onset of the first heart attack or
stroke, and we would like those data to be highly internally consistent. This has been an
issue for homocysteine evaluation, for which many prospective epidemiologic studies have
been positive but others have been less promising. But the third and most important
clinical criterion is the additive value of the new marker over and above standard
cholesterol screening. This has been the issue of interest for the inflammatory markers,
particularly fibrinogen and hs-CRP, because in fact these markers do appear to add to the
predictive value of standard lipid evaluation. Reference:
Ridker PM. Evaluating novel cardiovascular risk factors: can we better predict heart
attacks? Ann Intern Med 1999;130:933-937.
Web
site |
