| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |review |
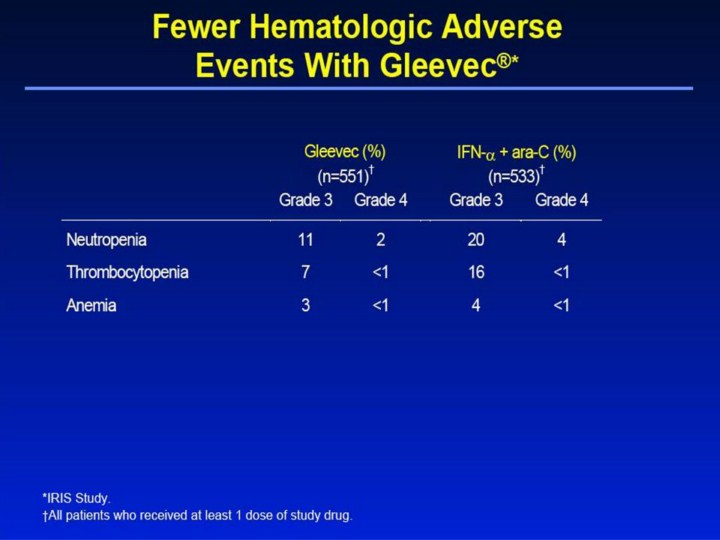 |
Fewer Hematologic Adverse Events With Gleevec®1,2 • Grade 3 neutropenia was observed in 11% of patients in the Gleevec group and in 20% of patients in the IFN-α + ara-C group. • Grade 3 thrombocytopenia occurred in 7% of patients in the Gleevec group and in 16% in the IFN-α + ara-C group. • Overall, myelosuppression was more severe in the IFN-α + ara-C group than in the Gleevec group. • Myelosuppression with Gleevec was also less severe in de novo patients compared with patients in chronic phase who failed IFN-α treatment (phase II trial, study 110). This difference may reflect a larger pool of normal hematopoietic stem cells in newly diagnosed patients.3 • Common Toxicity Criteria grades: neutropenia (grade 3 ≥0.5-1.0 x 109/L, grade 4 <0.5 x 109/L); thrombocytopenia (grade 3 ≥10-50 x 109/L, grade 4 <10 x 109/L); anemia (hemoglobin ≥65-80 g/L, grade 4 <65 g/L). References 1. Gleevec® (imatinib mesylate) Prescribing Information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2003. 2. Data on file. Novartis Pharmaceuticals Corporation, East Hanover, NJ. 3. Kantarjian H, Sawyers C, Hochhaus A, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346:645-652. |