| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |review |
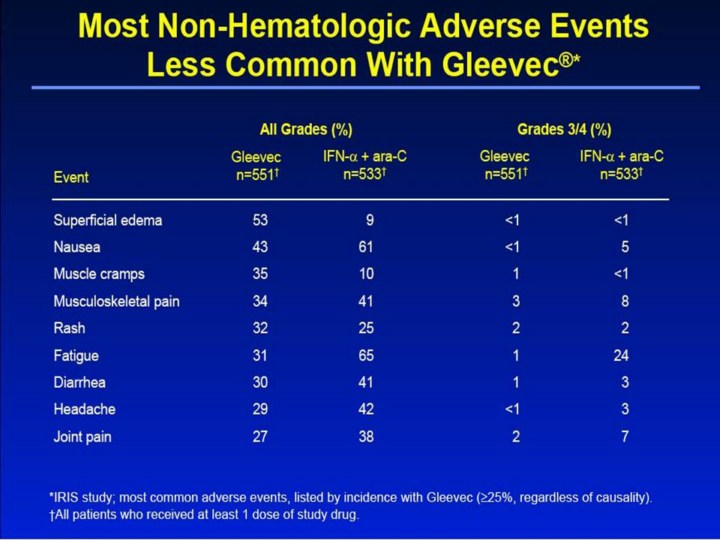 |
Most Non-Hematologic Adverse Events Less Common With Gleevec®1,2 • In the Gleevec group, most adverse events were mild to moderate (grades 1/2). The most common adverse events (all grades; ≥25%) were superficial edema (53%), nausea (43%), muscle cramps (35%), musculoskeletal pain (34%), rash (32%), fatigue (31%), diarrhea (30%), headache (29%), and joint pain (27%). • No severe (grades 3/4) non-hematologic event occurred in more than 3% of patients receiving Gleevec. • In the IFN-α + ara-C group, the most common adverse events (all grades; ≥25%) were fatigue (65%), nausea (61%), headache (42%), diarrhea (41%), musculoskeletal pain (41%), myalgia (39%), pyrexia (39%), joint pain (38%), depression (35%), rigors (34%), anorexia (31%), vomiting (27%), and rash (25%). • Severe (grades 3/4) fatigue, depression, myalgia, and musculoskeletal pain were observed in 24%, 12%, 8%, and 8% of patients receiving IFN-α + ara-C, respectively. • Severe lab abnormalities—including neutropenia (14%), thrombocytopenia (7%),anemia (3%), and hepatotoxicity (3%)—severe musculoskeletal pain (3%), fluid retention (including severe superficial edema) (<1%),* and hemorrhage (<1%) were reported among some Gleevec recipients. *Patients should be weighed and monitored regularly for signs and symptoms of edema, which can be serious or life threatening. References 1. Gleevec® (imatinib mesylate) Prescribing Information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2003. 2. Data on file. Novartis Pharmaceuticals Corporation, East Hanover, NJ. |