| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |review |
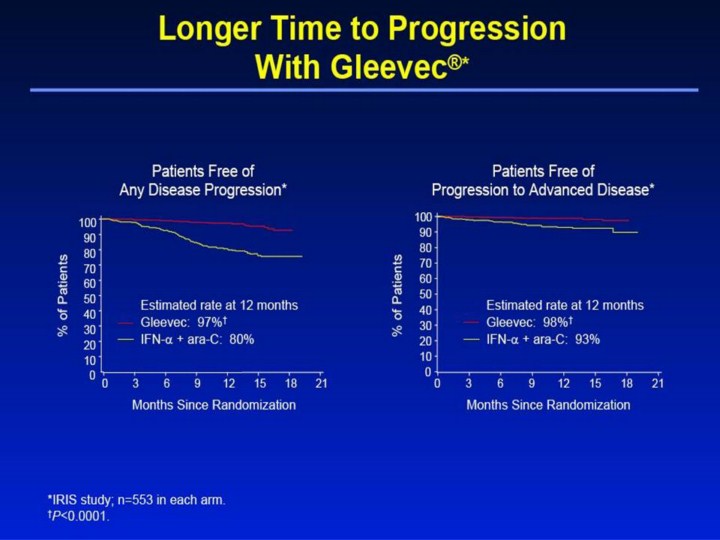 |
Longer Time to Progression With Gleevec®1,2 • Focusing on the primary endpoint of the study, this slide shows there was a significantly higher rate of progression-free survival (longer time to any disease progression) at 12 months with Gleevec, 97%, compared with 80% with IFN-α + ara-C (P<0.0001). • “Time to progression” is defined as the time between randomization and any of the following: progression to accelerated phase or blast crisis; death; loss of a complete hematologic response or major cytogenetic response; or in patients not achieving a complete hematologic response, an increasing white blood cell count despite appropriate therapeutic management. • Of note, this analysis uses a strict intent-to-treat principle that may overestimate the rate of progression-free survival for IFN-α + ara-C, as approximately 40% of the patients crossed over to Gleevec, which has been shown to be effective as second-line therapy for patients failing IFN-α therapy. • A significantly higher estimated percentage of patients receiving Gleevec (98%) did not progress to advanced phase CML at 12 months, versus 93% for the IFN-α + ara-C group (P<0.0001). References 1. Gleevec® (imatinib mesylate) Prescribing Information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2003. 2. Data on file. Novartis Pharmaceuticals Corporation, East Hanover, NJ. |