| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |review |
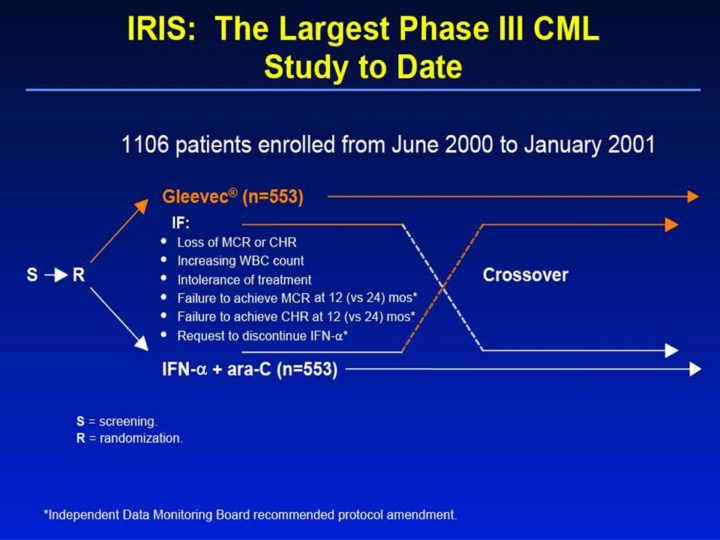 |
IRIS: The Largest Phase III CML Study to Date S S = screening. R = randomization. Gleevec® (n=553) IFN-α + ara-C (n=553) R Crossover IF: • Loss of MCR or CHR • Increasing WBC count • Intolerance of treatment • Failure to achieve MCR at 12 (vs 24) mos* • Failure to achieve CHR at 12 (vs 24) mos* • Request to discontinue IFN-α* *Independent Data Monitoring Board recommended protocol amendment. 1106 patients enrolled from June 2000 to January 2001 [slide 13] IRIS: The Largest Phase III CML Study to Date1,2 • From June 2000 to January 2001, 1106 patients were enrolled at 117 centers in 16 countries in the IRIS study (553 randomized to each treatment arm). This was the largest and most rapidly accrued phase III CML study to date. • The IRIS study (study 106) protocol allows for a crossover in the case of lack of response, loss of response, or intolerance of treatment. • After interim analysis of the trial data, the initial study protocol was amended by the Independent Data Monitoring Board (IDMB) to enable patients in either arm to cross over if no MCR had occurred after 1 year of treatment, instead of 2 years as initially required, and to enable patients in the IFN-α + ara-C arm to cross over to Gleevec® at any time, if desired. References 1. Gleevec® (imatinib mesylate) Prescribing Information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2003. 2. Data on file. Novartis Pharmaceuticals Corporation, East Hanover, NJ. |