| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |28 |29 |30 |31 |32 |33 |review |
 |
If you have any comments or questions, please send a
message to
super1@pitt.edu
C. Thomas Caskey, Annu. Rev. Med. 2007. 58:1–16 Portfolio Management Solutions
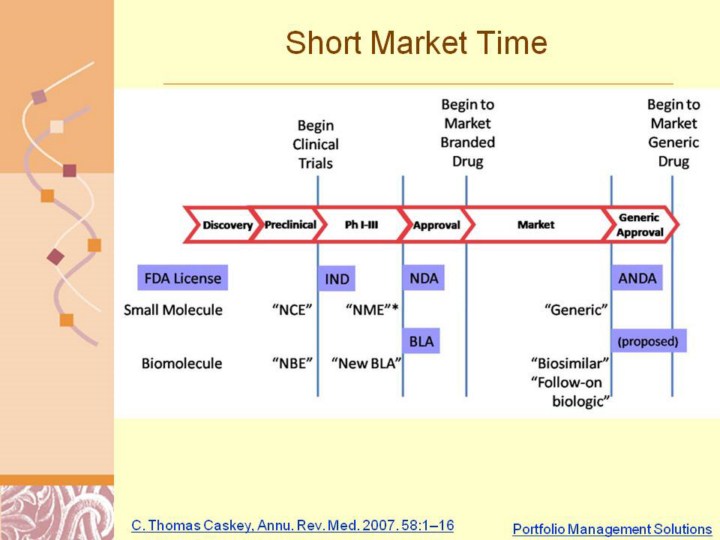
Figure 1, Small Molecules and Biomolecules can take on different names over the lifetime of drug discovery and development and marketing. Biosimilars are also referred to as Follow-on Biologics. Phase length is not implied by the size of stage marker. *NME relates to the first approvable drug as opposed to second indications or new formulations. (Ref 1) The application for a generic small molecule is an “Abbreviated New Drug Application” (ANDA) which doesn’t require clinical trials to prove equivalency. Processes for biosimilars or follow-on biologics are in the discussion stage.
|
Search inside of Supercourse and lectures in HTML or PPT format