| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |28 |29 |30 |31 |32 |33 |34 |35 |36 |37 |38 |39 |40 |41 |42 |43 |44 |review |
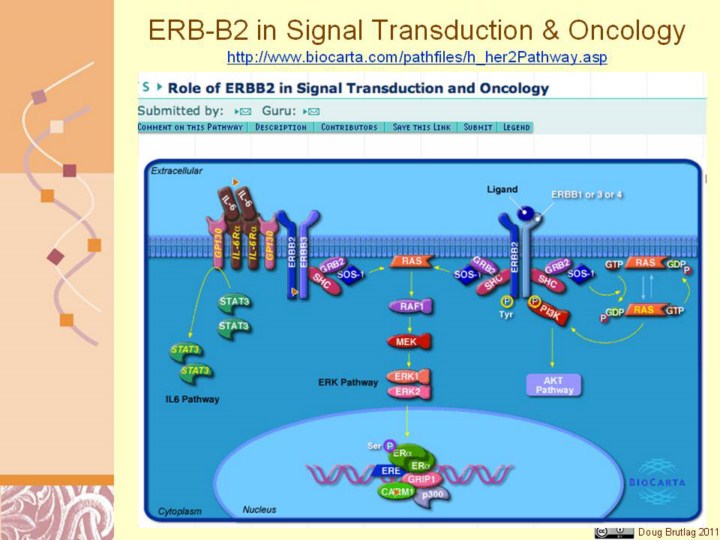 |
http://www.biocarta.com/pathfiles/h_her2Pathway.asp Her2 or ERBB2 belongs to a class of proteins having high homology with epidermal growth factor receptor (EGFR or ERBB1). It encodes a protein with the molecular weight of 185 KDa. Unlike other members of EGFR family, no ligand for Her2 has been found and it usually associates with members of ERBB1 family to form functional heterodimers. It has been shown that it can form dimers with ERBB (EGFR), ERBB3 and ERBB4 as well as gp130 subunits of IL-6 receptor. In at least some cell types, the association between gp130 and HRBB2 is essential for HRBB2-ERBB3 phosphorylation and subsequent MAP kinase signaling. Although ERBB1 can form homodimers, the signaling for ERBB1 is usually transient and the receptor undergoes internalization after ligand binding and activation. EGFR-HER2 complex increases the signaling capacity of EGFR by increasing the ligand affinity as well as the recycling of the heterodimer. Of all the ERBB heterodimers, ERBB2-ERBB3 heterodimers perhaps elicit the strongest signal. Removing ERBB3 from the cell has a drastic effect on ERBB2 mediated signaling and downstream effectors.
The clinical importance of HER2 cannot be overstated. In addition, monoclonal antibody (Herceptin) against this receptor has been shown to be an effective treatment of breast cancer patients who have a high level of HER2 over expression.
|