| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |28 |29 |30 |31 |32 |33 |34 |35 |36 |37 |38 |39 |40 |41 |42 |43 |44 |45 |46 |47 |48 |49 |50 |51 |52 |53 |54 |55 |review |
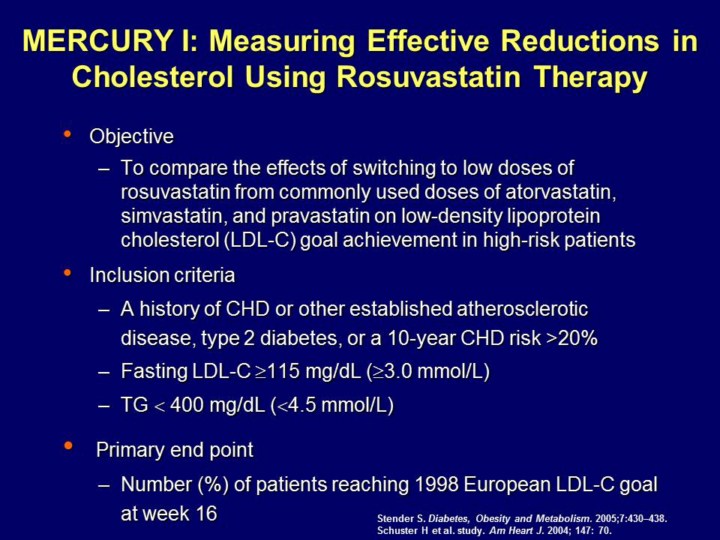 |
The MERCURY I trial was an open-label, randomized, multicenter, 5-arm, parallel-group switching study to compare the efficacy and safety of lipid-lowering agents atorvastatin, pravastatin, simvastatin and rosuvastatin in patients with hypercholesterolemia in Europe, Australia, and Canada.
Patients were required to have a history of CHD or other established atherosclerotic disease, type 2 diabetes or a 10-year CHD risk >20%; Fasting LDL-C 115 mg/dL, (³3.0 mmol/L) and TG 400 mg/dL (<4.5 mmol/L).
References: 1. Schuster H et al. Effects of switching statins on achievement of lipid goals: Measuring effective reductions in cholesterol using rosuvastatin therapy (MERCURY I) study. Am Heart J. 2004; 147: 705−712. 2. Stender S. Comparison of rosuvastatin with atorvastatin, simvastatin and pravastatin in achieving cholesterol goals and improving plasma lipids in hypercholesterolaemic patients with or without the metabolic syndrome in the MERCURY I trial* Diabetes, Obesity and Metabolism. 2005;7:430–438.
|