| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |28 |29 |30 |31 |32 |review |
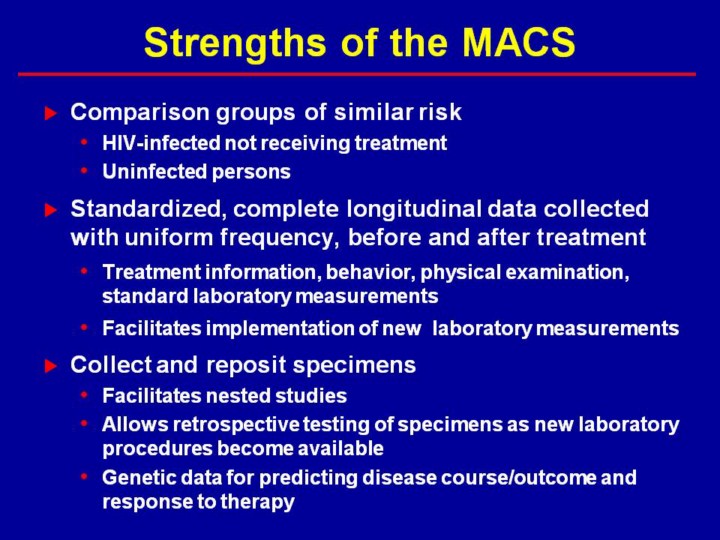 |
Observational and clinical cohorts provide different data as outlined above. Ideally data from both provide a more comprehensive picture of the natural history of treated disease. Observational cohorts, however, also provide data on the natural history of untreated disease which provides a comparison and can also provide predictors of response to therapy. Observational cohorts also provide data on comparable groups of uninfected individuals for determination of background rates of diseases that may be increased by therapy. Clinical cohorts provide a “real world” approximation of drug regimens among treated patients, but observational cohorts provide a “real world” approximation of population effectiveness of therapy because they include both patients in therapy and those not in therapy. Clinical cohorts can provide a broader range of routine laboratory tests but do not provide the same level of consistency and quality control of laboratory tests possible in observational cohorts and are dependent on tests that are performed consistently across all the hospitals/clinics. New laboratory procedures are more easily implemented uniformly in observational cohort studies. Observational cohort studies have the important advantage of providing a repository for retrospective testing with new laboratory tests and, thus, facilitate implementation of nested case-control studies. Observational studies require an infrastructure for collection of data and laboratory testing, but do not require the infrastructure for abstraction and collation of diverse data from multiple hospitals/clinics. Thus, it is appropriate to consider observational and clinical cohorts as providing complementary data for understanding HIV/AIDS.
|