| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |28 |29 |30 |31 |32 |33 |34 |35|36 |37 |38 |39 |40 |41 |42 |43 |44 |45 |46 |47 |48 |49 |50 |51 |52 |53 |54 |55 |56 |57 |58 |59 |60 |61 |review |
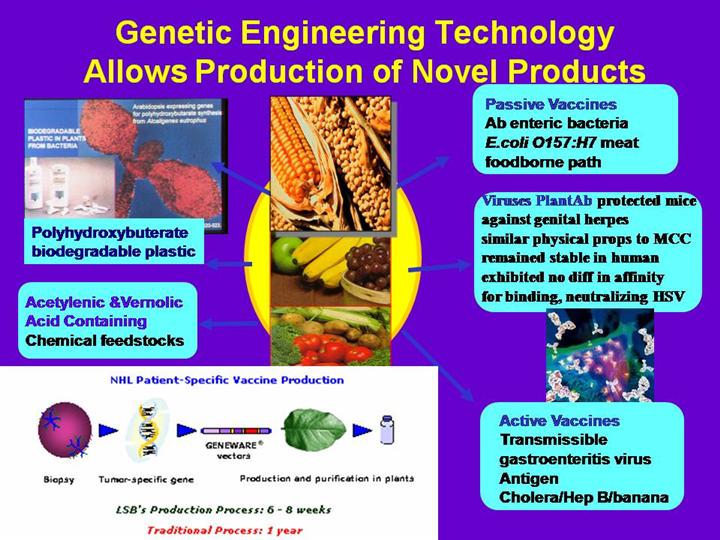 |
ANTIBODY-CONTAINING SOYBEANS During the early 1990s, researchers discovered that special vaccination of flocks of chickens caused them to secrete antibodies [against the bacterial strains chosen for vaccination] into the whites of the eggs they laid. Those egg whites are now chopped-up to prepare a commercial piglet feed that removes all E. coli bacteria of specific diarrhea-causing strains from the intestines of piglets. xx That egg-white-based feed product works via each antibody “latching onto” one of the diarrhea-strain-specific E. coli bacteria within the piglet’s digestive system, then the combined pair is excreted by the animal. Because antibodies are pure protein molecules, there are no regulatory issues pertaining to meat residues.
Today’s periodic outbreaks of beef-borne E. coli 0157:H7 bacterial disease occur because cattle became tolerant of E. coli 0157:H7 in the 1970s [it had previously killed infected cattle] xxi and humans are now sometimes exposed to that deadly bacteria when the hide or digestive system contents of cattle come into contact with meat (e.g., at slaughterhouses). xxii A study published by USDA in April, 2000 showed that reducing E. coli 0157:H7 in live cattle prior to slaughter greatly increases slaughterplant safety. xxii It is now possible for biotechnology to cause specific antibodies (e.g., specific to E. coli 0157:H7) to be produced in soybeans, so such future soybeans could be fed to livestock for 72 hours prior to slaughter in order to eliminate outbreaks of foodborne diseases such as E. coli 0157:H7, Salmonella spp, etc.
12. “VACCINE-CONTAINING” SOYBEANS AND MAIZE During 2001, a U.S. company will introduce a biotechnology derived maize that produces antigens for the swine disease known as transmissible gastroenteritis virus (TGEV). When that maize is eaten and those antigens in it tough lymph tissues in the swine’ digestive system, the animals’ immune system rapidly produces antibodies that protect it against TGEV. Similar plant vaccines are expected in the future for human diseases such as hepatitis B. Medical Benefits Plants have been a valuable source of pharmaceuticals for centuries. During the past decade, however, intensive research has focused on expanding this source through rDNA biotechnology. The research brings closer to reality the prospect of commercial production in plants of edible vaccines and therapeutics for preventing and treating animal and human diseases. Possibilities include a wide variety of compounds, ranging from vaccine antigens against hepatitis B and Norwalk viruses (Arntzen, 1997; Dixon and Arntzen, 1997; Mason et al., 1992, 1998) and Pseudomonas aeruginosa and Staphylococcus aureus (Brennan et al., 1999) to vaccines against cancer and diabetes. In addition, genetically modified strains of probiotic microorganisms are also possible vehicles for successful delivery of vaccines and digestive aids (e.g., lactase) through the stomach and the small intestine. Two seminal papers supported the use of rDNA biotechnology-derived plants for pharmaceutical production (Ma et al., 1995, 1997). These reports were soon followed by one (Ma et al., 1998) describing results of successful human clinical trials with an edible vaccine against a pathogenic strain of E. coli and a monoclonal antibody against cariogenic Streptococcus mutans. Haq et al. (1995) reported the expression in potato plants of a vaccine against E. coli enterotoxin against the toxin in mice. Human clinical trials suggest that oral vaccination against either of the closely related enterotoxins of Vibrio cholerae and E. coli induces production of antibodies that can neutralize the respective toxins by preventing them from binding to gut cells. Ma et al. (1995, 1998) showed that tobacco plants could express secretory antibodies or “plantibodies” against the cell surface adhesion protein of S. mutans. Used as a bactericidal mouthwash, the antibodies prevented bacterial colonization by the microorganism and development of dental caries for four months. A similar approach showed that soybean-produced antibodies protected mice against infection by genital herpes (Zeitlin et al., 1998). Compared to antibodies produced in mammalian cell culture, the plantibodies had similar physical properties, remained stable in human reproductive fluids, and exhibited no differences in their affinity for binding and neutralizing herpes simplex virus. Hence, the difference in the glycosylation processes of plants and animals does not appear to affect the immune functions of the plant-derived antibodies. Non-Hodgkins B-cell lymphoma, the most widespread cancer of the lymph system, is difficult to treat because the B-cell tumors are variable and response to treatment can vary from person to person. Hence, effective therapy requires “personalized medicine” tailored to the genetic makeup of each patient’s tumor. Unfortunately, conventional treatment methods do not meet the needs for rapid production of customized antibodies in sufficient quantities. Monoclonal antibodies used in conventional treatment also tend to be expensive and unreliable, and those produced in bacteria have solubility and conformation problems. A system using tobacco mosaic virus (TMV) was developed to produce in tobacco plants (Nicotiana benthamiana) a therapeutic vaccine against non-Hodgkin’s B-cell lymphoma in a mouse model (McCormick et al., 1999). Using cells cloned from malignant B-cells of mice, TMV DNA was modified with a tumor-specific sequence from the gene coding for the immunoglobin cell surface marker. Plants were then infected with the modified virus, resulting in expression of cancer-specific antibodies. B-cell proteins were then extracted from the plant leaves for vaccination of the mice. Eighty percent of the mice receiving the while all untreated mice died within three weeks of contracting the disease. A similar approach was used to develop a vaccine against insulin-dependent diabetes mellitus (IDDM), an autoimmune disease in which insulin-producing cells of the pancreas are destroyed by the cytotoxic T lymphocytes. The “oral tolerance” method of preventing or delaying autoimmune disease symptoms involves the ingestion of large amounts of immunogenic proteins that turn off the autoimmune response. This method of vaccination is gaining recognition as a potential alternative to systemic drug therapy, which is often ineffective. Insulin and pancreatic glutamic acid decarboxylase (GAD), which are linked to the onset of IDDM, are candidates for use as oral vaccines. Blanas et al. (1996) described the development in a mouse model of a potato-based insulin vaccine that is almost 100 times more powerful than the existing vaccine in preventing IDDM. Feeding diabetes-prone mice potatoes engineered to produce immunogenic GAD reduced the incidence of disease and immune response severity. rDNA biotechnology-derived vaccines are potentially cheap, convenient to distribute, and simple and safe to administer. Production of medically important substances via rDNA biotechnology engineering of plants and microorganisms offers multiple advantages. For plants, production can be done virtually anywhere and has the potential to address problems associated with delivery of vaccines to people in developing countries. Products from these alternative sources do not require a so-called “cold chain” of refrigerated transport and storage, although they will require segregation from conventional foods to prevent inappropriate consumption. Pharmaceuticals or therapeutics produced via genetic engineering of plants also offer an alternative delivery method, feeding versus injection (Howard, 1999), and an alternative to extraction from animal sources. Furthermore, rDNA biotechnology-derived vaccines may also be safer than many conventional vaccines because they consist of pathogen or antibody subunits rather than whole microorganisms. The use of plants can facilitate abundant production of therapeutic proteins without the risk of contamination by animal pathogens, and at substantially reduced cost. Biotechnology Report: Benefits C O N T I N U E D |