| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |28 |29 |30 |31 |32 |33 |34 |35 |36 |37 |38 |39 |40 |41 |42 |43 |44 |45 |46 |47 |48 |49 |50 |51 |52 |53 |54 |review |
 |
Although a
cure for T1D is currently unavailable, several large international
investigations have been designed to evaluate a number of primary and
secondary disease interventions.
Two are targeted towards unaffected first degree relatives (FDR) in
families with an individual with T1D.
One is targeted to the general population (GP) of Finland.
To determine if individuals are eligible, genetic testing for
DQB1 is performed.
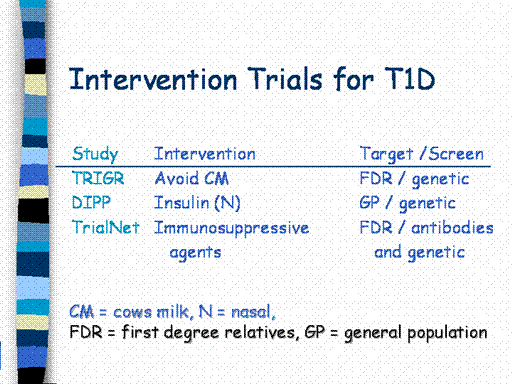
For the Trial
to Reduce Type 1 Diabetes in Genetically at-Risk (TRIGR) and the
Diabetes Prediction and Prevention Project (DIPP), genetic testing is
performed as part of newborn screening.
Infants who carry high risk DQB1 alleles are eligible to
participate. For TRIGR, newborns
are randomized to receive either a regular cow’s milk formula or one
with hydrolyzed proteins, which is thought to be protective.
This study is being conducted in Europe and North America.
For DIPP, newborns with high risk DQB1 alleles from the general
Finish population are followed until they develop beta cell antibodies.
When this occurs, they are randomized to an intervention based on
nasal insulin.
New
interventions that will be tested in the future will be conducted
through T1D TrialNet, a collaborative network of clinical centers and
experts in diabetes and immunology.
These studies will identify unaffected first degree relatives
with beta cell autoantibodies who will be eligible for new
interventions. Those who carry
the protective DQB1*0602 gene, however, are generally excluded. The
first TrialNet intervention study is testing two immunosuppressive
agents (Mycophenolate mofetil) alone or in combination with Daclizumab.
|