| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |review |
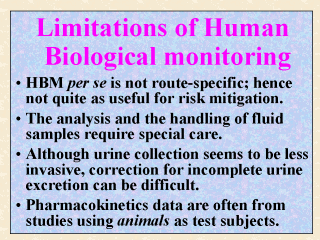 |
The advantages
of using the biomonitoring techniques as discussed in the last slide are not free. The
limitations are likewise numerous. The total body burden derived from this type of studies
is not route-specific, unless further effort is made such as collecting air samples on the
side to separate the dose acquired from inhalation exposure. For risk mitigation purposes,
it is always important to know which route of entry is the significant exposure pathway.
If dermal exposure for the worker is found to be much more significant than from
inhalation, then the worker shall be required to wear adequate protective clothing,
instead of an approved respirator. The ethical issues were discussed in Lecture 4, so were those on the sensitivity and the specificity of the analytical method used. Also, the analysis and handling of fluid samples require especial care. For example, whole blood samples should never be frozen (U.S. EPA, 1998). Collection of urine over 24 hours or longer is seldom practical. Endogenous urinary creatinine levels are hence often used to correct for incomplete urine excretion. Yet as pointed out in Dong et al. (1994), urinary creatinine levels are not always useful for calculating the daily urine outputs since the excretion rate of creatinine varies with time and some endogenous factors (see next slide for further discussion). Although doses derived from well-designed biomonitoring studies can reduce the uncertainty with animal dermal absorption, the back-calculation as illustrated in Slide 15 requires urinary recovery data from pharmacokinetics studies conducted on animals rather than on humans. |