| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |review |
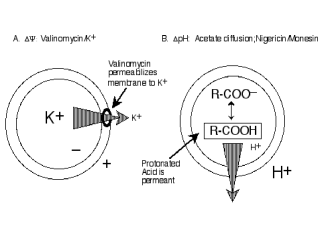 |
Figure 10. Other ways to energize active
transport in addition to D-lactate oxidation. (a) Artificial electron donors
[ascorbate/phenazine methosulfate (ASC/PMS); dithiothreitol/ubiquinol-1];
anaerobic electron transfer to nitrate or fumarate; (b) Internal ATP
hydrolysis; (c) Artificially imposed membrane potentials (∆Ψ)
or pH gradients (∆pH). Left. Vesicles loaded with KPi are diluted into NaPi, and valinomycin, a K+-specific ionophore (see Lecture II) is added to make the membrane specifically permeable to K+ (like the resting potential in a nerve cell). Since Na+ and Pi are impermeant, an outwardly directed K+ flux generates a ∆Ψ (interior negative) that will drive accumulation of various transport substrates. Right. Vesicles loaded with acetate are diluted into buffer containing an impermeant acid like gluconate. Since only the protonated (uncharged) form of acetate permeant, acetic acid leaves the vesicles, depleting the inside of protons and generating a ∆pH (interior alkaline). pH Gradients can also be generated with the ionophores nigericin which has a higher affinity for K+ than Na+ or monensin which has a higher affinity for Na+ than K+. As opposed to valinomycin, these ionophores catalyze the 1:1 exchange of protons for either K+ or Na+ across the membrane. Thus, vesicles containing one of these ionophores and loaded with cholinePi are diluted into either KPi or NaPi. K+ or Na+ influx then drives efflux of protons out of the vesicles with generation of ∆pH (interior alkaline). The reverse ∆Ψ or ∆pH can aslo be generated by reversing the gradients. |