 |
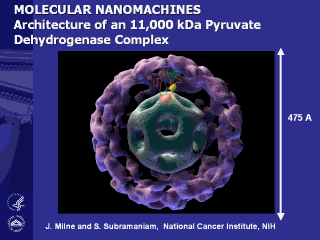
Sectioned schematic view of an icosahedral
pyruvate dehydrogenase complex based on electron cryo-microscopic analysis
of an E1E2 sub-complex from Bacillus stearothermophilus. Three of the 60 E2
molecules (colored red, green, and yellow) are highlighted. The movement of
the swinging E2 lipoyl domain in the annular region between the inner core
(cyan) of E2 molecules and the outer shell of 60 E1 molecules (purple) is
proposed to be a critical feature underlying active site coupling in the
complex. The pyruvate dehydrogenase complex constitutes one of the
cornerstones of cellular energy metabolism, mediating the oxidative
decarboxylation of pyruvate to generate acetyl CoA, linking glycolysis and
the tricarboxylic acid cycle.
|
