|
The contraction of the myocyte results from the
spike in cytoslic free Ca2+ which precedes the contraction spike by some 50
ms. The time-averaged cytosolic free Ca2+ levels remain very low, normally
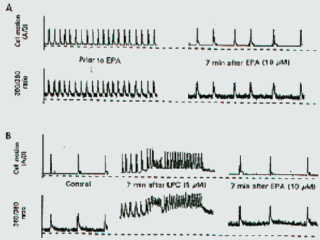
circa 100 nM. EPA reduced the beating rate without altering the amplitude of
contractions, as reported. On another myocyte, which had a much slower
endogenous beating rate, Fig. 8B shows the effect of LPC (15
µM) on increasing the cytosolic free
Ca2+ concentrations and fluctuations and the resulting tachyarrhythmia. The
presence of EPA (10 µM) added to the
superfusate reduced the cytosolic [Ca2+]i, sufficiently to terminate the
tachyarrhythmia, though not to normal concentrations in this experiment.
This beneficial effect of EPA to terminate the arrhythmia results from the
action of PUFA to inhibit the L-type Ca2+ current (ICa,L), in adult rat
cardiomyocytes is shown in Fig. 9. Such excessive
cytosolic free Ca2+ fluctuations, as shown in Fig 8B after LPC can induce
delayed after-potentials. If the after-potentials become of sufficient
magnitude they may activate Na+ channels to initiate aberrant action
potentials. If these occur at a vulnerable moment in the electrical cycle of
the heart, fatal arrhythmias may occur. Extracellular application of EPA and
the other antiarrhythmic polyunsaturated fatty acids, but not saturated or
monounsaturated fatty acids produced a prompt and reversible
concentration-dependent inhibition of ICa,L.
|
