| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |28 |29 |30 |31 |32 |33 |34 |35 |36 |37 |review |
 |
Vaccines are produced each year for seasonal
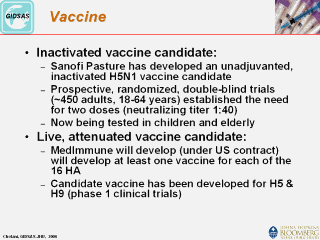
influenza but will not protect against pandemic influenza. According to the WHO although a vaccine against the H5N1 virus is under development in several countries, no vaccine is ready for commercial production and no vaccines are expected to be widely available until several months after the start of a pandemic. Some clinical trials are now under way to test whether experimental vaccines will be fully protective and to determine whether different formulations can economize on the amount of antigen required, thus boosting production capacity. Because the vaccine needs to closely match the pandemic virus, large-scale commercial production will not start until the new virus has emerged and a pandemic has been declared. Current global production capacity falls far short of the demand expected during a pandemic. Two candidate vaccines have been developed and are currently being tested. |