| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |review |
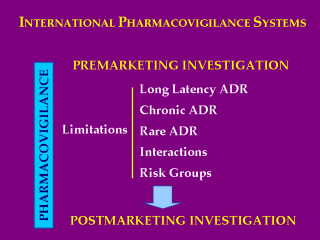 |
These events lead
to a crescent involvement of governmental authorities on drug safety
regulation, developing National and International Pharmacovigilance Systems. Pharmacovigilance is crucial in pre and post marketing investigation, post marketing surveillance acquiring enormous importance due to the limitations of clinical trials in the detection of adverse drug reactions. (ADR) |