 Click for
larger picture |
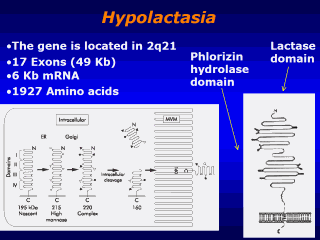
Model of the
molecular forms of lactase-phlorizin hydrolase during synthesis and
processing in the human villus enterocyte. The early changes in apparent
molecular size are due to glycosylation, as indicated in the diagram. Note
that the two active sites are located in domains III and IV. The
subsequently removed domains I and II are important for correct folding of
the nascent protein. Although not indicated on this drawing, the enzyme
forms a homodimer during processing. The final N terminal cleavage of a
small segment is depicted by the elimination of the terminal loop in the
microvillus form of the enzyme. these two human phenotypes. The gene for
human LPH, located on chromosome 2q21, comprises 17 exons and covers
approximately 49 kb, giving rise to a messenger RNA (mRNA) of slightly more
than 6 kb. From initiation codon to stop codon, human LPH mRNA
encodes 1927 amino acids forming the complete translation product. Sequence
comparisons indicate that the coding region is comprised of four homologous
parts, leading to the suggestion that the gene is the product of two
duplication events during evolution. The nascent protein is heavily
glycosylated so that the final translation product is about 220 kDa (fig).
This high molecular mass glycoprotein undergoes intracellular cleavage,
dividing regions I and II from regions III and IV. The protein consisting of
regions III and IV contains the two active sites and is inserted into the
microvillus membrane of the enterocyte as a mature enzyme of approximately
160 kDa. The proximal portion encompassing regions I and II has no enzymatic
activity, but has been shown to function in correct folding of the enzyme.
|
