| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |28 |29 |30 |31 |32 |33 |34 |35 |36 |37 |38 |39 |40 |41 |review |
 |
There are
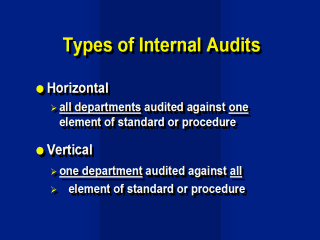
generally two types of audits: Horizontal Audits In this approach the audit program is based on periodic audits directed at the application of particular elements across the organisation. One element is chosen, for example document control, and the application of this element is examined in all departments eg. document control would be audited in Serology, Virology and Administration. This type of audit is useful in identifying differences in the interpretation or application of quality system procedures between different parts of the organisation. A horizontal audit does not address the interaction between different procedures or processes. Vertical Audits In this approach a particular test or process is chosen and all inputs, operations and activities that go into conducting the test are examined i.e all elements of the standard are reviewed in one department. For example, with an EIA test all steps which go towards producing a results are examined, from when the sample was first booked into accession to when the report was released. The most practical way to conduct a vertical audit is to choose a test report and follow the records and procedures back through the system to ensure all activities were adequately completed. Reagents, equipment, staff skills, procedures and document control are all reviewed in relation to this sample. A vertical audit examines the interfaces between all the systems elements and procedures but may miss the inconsistent application of procedures between different activities. |