| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |28 |29 |30 |review |
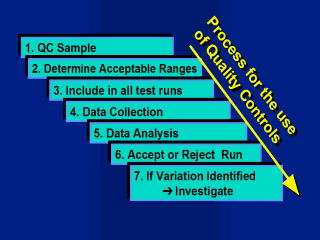 |
The QC sample
process 1. QC Sample: A large volume of QC sample previously prepared or chosen, must be adequately labelled and stored. 2. Determine Acceptance ranges: Ranges for accepting and rejecting runs need to be determined by testing the sample a number of times within the laboratory. Initially the sample may be tested on a minimum of 20 samples to establish and initial mean and limits. As data accumulate over time the acceptance ranges will need to be recalculated. 3. Testing: The QC sample should be tested in all assay runs. 4. Data Collection: Enter the data on a daily basis from each run of the external QC sample. For an external QC programme results must be collected and validated. 5. Data Analysis: After each run results are graphed to identify any shifts or trends in variation, results are analysed by comparing to acceptable ranges. 6. Accept or Reject Run: The run is accepted if the QC results fall within the predetermined acceptance range. The run is rejected and run repeated if the QC result is outside the range. This is in addition to the verification of the run using internal kit controls 7. Investigate if Variation Identified: If the run fails or variation is identified due to control charting or statistical analyses, elements of the test system may need to be investigated. An external QC program will require a more formalised and structured approach involving the distribution of sample and the collection of data from its participants. Feedback should be provided to participants in regular reports which are based on statistical analyses. |