| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |28 |29 |30 |review |
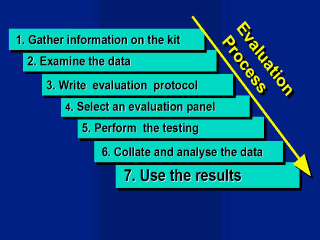 |
Evaluation
Process:
If evaluations are to be performed a process must be followed. Information on the kit or assay can be obtained from the manufacturer. Manufacturers usually have a large amount of information available. While this information is confidential and will not be shared with all laboratories if a regulatory process is in place, the information will be made available to the reference laboratory. An evaluation panel is selected. Suitable samples to form the evaluation panel are assembled. Appropriate documentation is carried out. Performing the testing |