| front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |review |
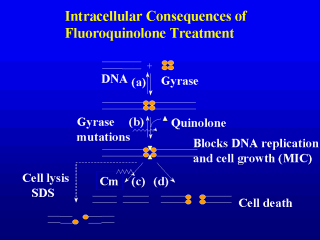 |
In order to describe the origins
of the MPC idea, it is useful to consider how the fluoroquinolones work (for review see
(10)). The central idea is that blocking cell growth is different from lethal action. DNA
gyrase and DNA topoisomerase IV, the targets of the quinolones, are homologous enzymes
that act by breaking DNA, passing a portion of double-stranded DNA through the break, and
then resealing the break. Step a shows gyrase binding to DNA and introducing
a pair of single-strand breaks that are staggered by 4 bp. In step b the quinolones
trap a reaction intermediate in which the DNA is broken (complexes can also form when only
one strand or neither stand is broken). A ternary complex arises that contains drug,
broken DNA, and topoisomerase. This complex does not form if gyrase or topoisomerase IV
contain mutations that reduce the ability of the quinolone to bind to the protein. The
ternary complexes block the movement of replication forks and transcription complexes,
thereby inhibiting cell growth. However, complex formation is reversible, and so it does
not account for cell death. We believe that cell death occurs when double-strand DNA breaks in the complexes are released from the complexes (3). This can occur in two ways, both of which are currently hypothetical. One (pathway c) involves induction of a suicide protein(s). In this pathway chloramphenicol, an inhibitor of protein synthesis, blocks the lethal action of quinolones, especially the older compounds such as nalidixic acid and oxolinic acid. The newer fluoroquinolones, such as ciprofloxacin, retain some lethal activity in the presence of chloramphenicol. Consequently, there must be another lethal pathway. We have proposed that this second pathway involves the dissociation of the topoisomerase subunits (3). The important point is that blocking cell growth is not the same as killing cells. |