|
|
|
|
front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |review |
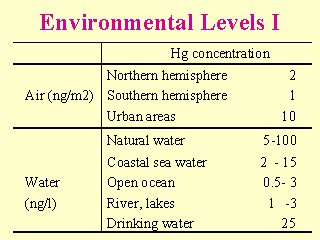 |
Some mercury will be present in the environment, because
it is a naturally occurring element. Nearly all mercury
is produced from the sulfide ore cinnabar (HgS) by means
of roasting the ore in air. The reaction is :
(Note: ng/m3 or ng/litre = 1ppt = 0.001 ppb) (WHO 1990) |