Search for most updated materials ↑
 |
 |
front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |28 |review |
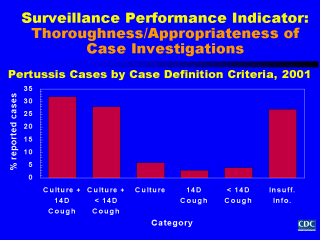 Performance indicators for thoroughness and appropriateness of case investigations can also be defined by aspects of the case definition used to identify suspected cases of a particular disease. Assessments of these performance indicators help us understand if information needed to confirm suspected cases of diseases is being collected properly, and if so, how well it is being done. In addition, if these indicators are examined for each case reporting site or source over a series of years, the results will indicate the comparability of the reported information between different sites and across different years. Let’s see how applying aspects of the case definition for pertussis can inform us about the thoroughness and appropriateness of the case investigation process for a pertussis surveillance system. First, let’s assume that the case definition for pertussis is: A cough illness lasting at least 2 weeks with paroxysms of coughing, inspiratory "whoop", or post-tussive vomiting without other apparent cause. Laboratory criteria for diagnosis and confirmation of pertussis disease include isolation of B. pertussis from a clinical specimen, or positive polymerase chain reaction (PCR) assay for B. pertussis. In this graph, data concerning the cases of pertussis reported to our surveillance system have been classified according to combined information on laboratory testing and cough duration needed to rule in or out a confirmed case of pertussis disease. As the data summary reveals, adequate laboratory and cough duration information was collected to determine the case status for 60% of cases. Of concern, is the 6% of suspected cases for which a culture but not information on cough duration was obtained, the 7% of suspected cases for which cough duration but not a culture was obtained, and the 27% of suspected cases for which there was insufficient information concerning cultures and cough duration. Theses findings suggested that our surveillance system needed improvement, particularly regarding collection of cultures and cough duration information during case investigations. By looking at other elements (e.g., completeness and timeliness of case reporting; date of onset, duration, and type of other clinical symptoms; number of case contacts identified and treated in order to prevent or limit the spread of disease; vaccination history information including dates of vaccination, and vaccine type, manufacturer, and lot number), we can identify additional areas where surveillance for pertussis disease can be improved. As before, the types of performance indicators used to assess surveillance for infectious and vaccine-preventable diseases can also be used to assess practices and procedures focused on surveillance of exposures to environmental and occupational toxicants. Performance indicators used to assess surveillance for chronic diseases and injuries could focus on thorough and appropriate collection of information concerning participation in behaviors which reduce and/or increase risk for development of disease or occurrence of accidents. |
 |
 |
front |1 |2 |3 |4 |5 |6 |7 |8 |9 |10 |11 |12 |13 |14 |15 |16 |17 |18 |19 |20 |21 |22 |23 |24 |25 |26 |27 |28 |review |