Chemistry Dept. Spring, 1999

![]()

Table of contents:
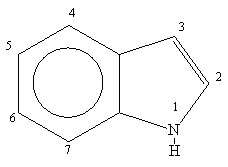

The indole ring is a versatile heterocyclic system which is used by synthetic and medicinal chemists in the construction of many natural products and neurochemicals.
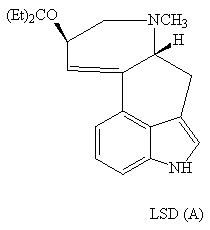
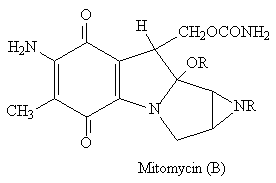
The indole ring is found in a range of compounds from LSD (A) psycho-actives to antibiotics such as mitomycin (B).
The parent indole ring was first prepared by zinc dust distillation of oxindole in 1866. The word indole is derived from India since the blue dye indigo was obtained from that country as early as the 16th century.
Indole compounds play an important role in fundamental
metabolism since it is found in the essential amino acid tryptophan.
I hope that the following information on synthetic approaches to the indole ring is useful to you. This web page will be under constant construction , so check back periodically for changes.
If you know of indole synthetic methodology not included be sure to email me and I will add the information to this web page.

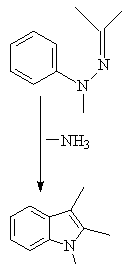
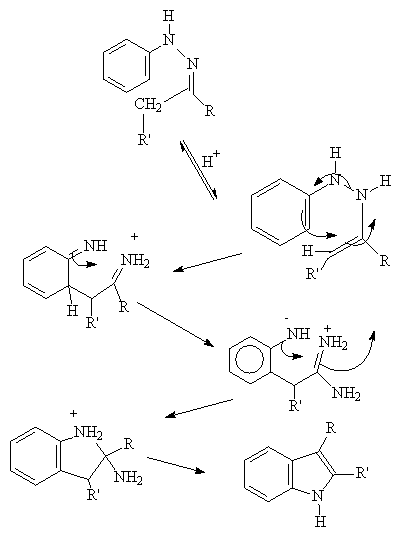
THE REACTION PROCEEDS VIA A 3,3, SIGMATROPIC SHIFT:
Acid catalysts include PPA , PCl3, HCl, ZnCl2
The reaction cannot be used to prepare indole rings unfunctionalized
in the 2 position, which is unfortunate since these derivatives are in
very high demand in the pharmaceutical industry as starting materials in
selected syntheses.
References:
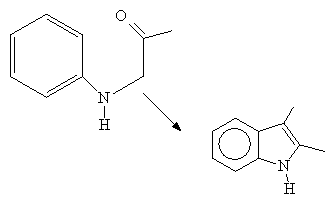
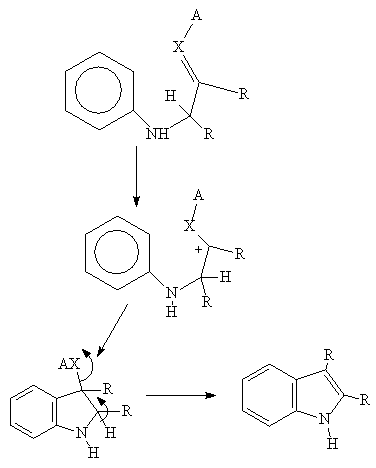
The reaction does not work with electron withdrawing groups on the aryl ring. The reaction is conducted in the presence of an acid catalyst which enhances the electrophilic character of the carbonyl carbon of the amide. Cyclization occurs at the point ortho to the aniline nitrogen:
Ex:
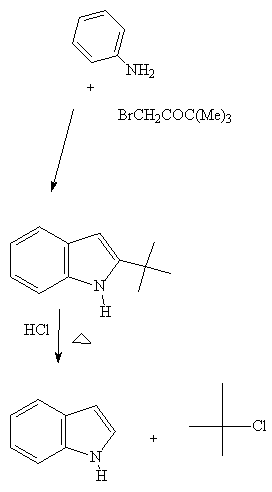
See: C.Abs. 50, 10709, 1956
This is an interesting example because it illustrates the possibility
Of cyclization followed by removal of the t-butyl group
at the 3 position. This reaction, so sar, has not been exploited for the
synthesize of A- ring oxygenated indoles.
References on the Bischler reaction:
3. The Reissert Reaction and its Variations
A very useful synthetic method which can afford a wide range of indoles with various groupings positioned on the indole ring.
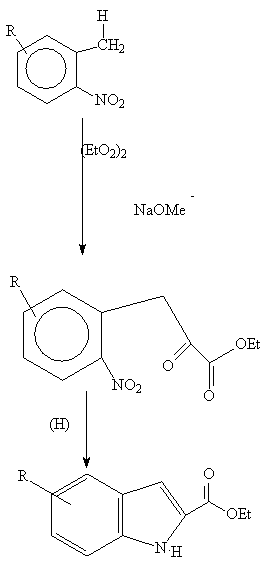 An ortho –toluene
is condensed with oxalate to give an intermediate (1) which, on reduction,
yields an indole 2- carboxylic acid which can be decarboxylated :
An ortho –toluene
is condensed with oxalate to give an intermediate (1) which, on reduction,
yields an indole 2- carboxylic acid which can be decarboxylated :
(1)
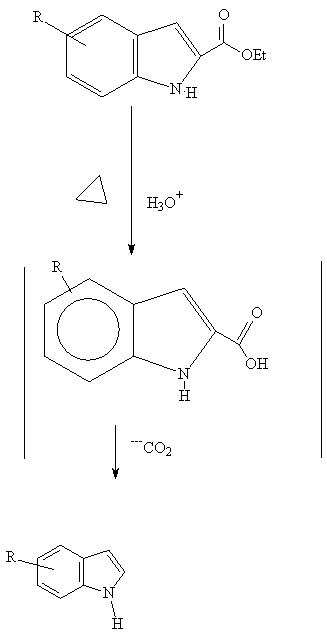
Hydrolysis and heating leads to the indole derivative (2):
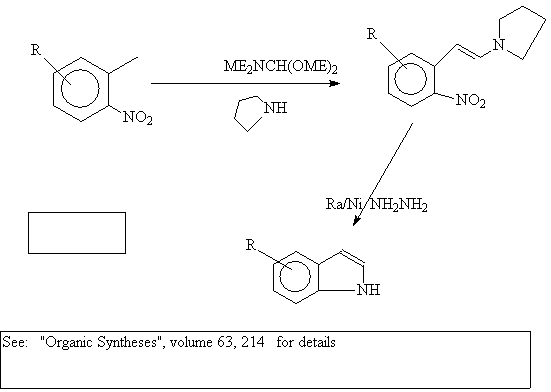
A important variation of this route was developed by Batcho
and Leimgruber at Hofmann- LaRoche:
References:
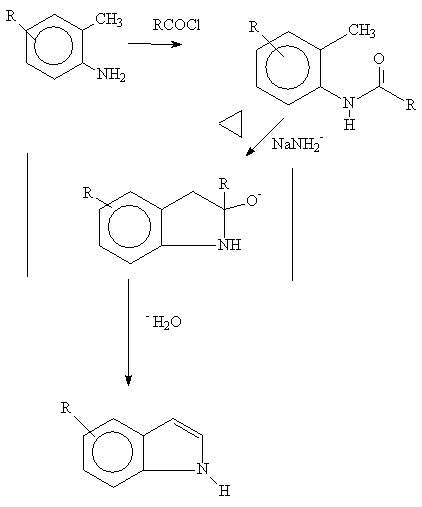
Generally , strong base such as sodamide, or potassium ethoxide, is used at 250-300 degrees C. Presumably, a proton is pulled from the benzylic carbon followed by nucleophilic attack on the carbonyl group. Dehydration forms the indole system.
R groups, carried on the A ring, must be insensitive to
these forcing conditions.
References:
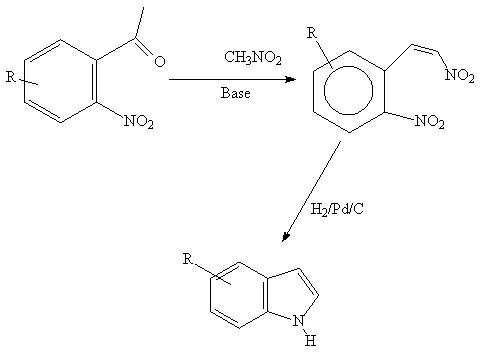
A versatile method which depends on the availablity of
the functionalized 0- nitrobenzaldehydes.
References:
These are the major synthetic entries into the indole ring system.
Additional minor approaches will be added at a later date.