Materials Science
Peptide Nucleic Acids
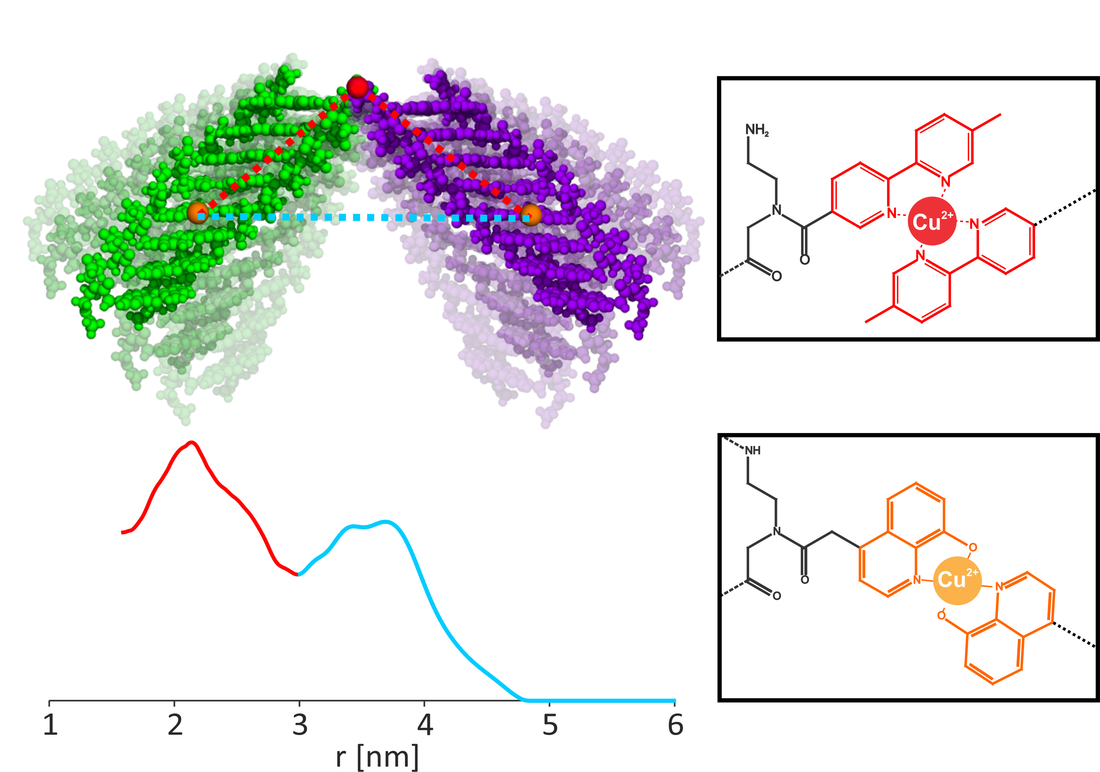
Peptide Nucleic Acids, or PNA, are a class of DNA analogues in which a polypeptide replaces the typical sugar-phosphate backbone found in other nucleic acids. This unique backbone imparts useful physical and chemical properties on the PNA chain, which can form duplexes with complementary strands of PNA or DNA. These properties include higher thermal and biological stability and greater sensitivity to mismatched base-pairs, making PNA an attractive option for the development of three-dimensional architectures for drug delivery, biological sensors and nanomachines.
In collaboration with the Achim group at Carnegie Mellon University, we seek to guide the development of simple, intuitive building blocks for such purposes by bridging short, modular PNA duplexes using metal ions and metal-binding ligands. By using Cu(II) as our bridging metal, we can use Electron Spin Resonance to measure distance constraints and determine the relative orientations of each PNA duplex. Additionally, ESR can assess the efficiency of the bridging mechanism and help us determine ways to optimize the system.
Collaborator: Prof. Catalina Achim (CMU)
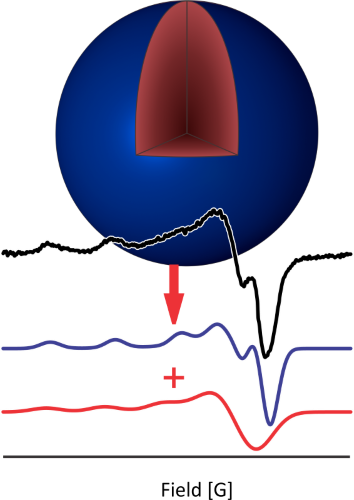
Nanoparticles
Cu2–xSe nanoparticles are part of a promising class of nanomaterials where the location, stability, and structural impact of charge carrier generation are crucial to their optoelectronic properties. In collaboration with the Millstone group at Pitt, we use electron paramagnetic resonance to analyze the oxidation of Cu(I) to Cu(II) that occurs in these nanoparticles when exposed to air. EPR allows us to identify the location and chemical environments of the generated Cu(II) and elucidate the dynamics of Cu oxidation. The impact of this oxidation provides us insight into the overall particle crystallographic structure and optoelectronic properties.
Collaborator: Prof. Jill Millstone (Pitt)
